One of the most widely used methods of analysis is the measurement of light. By irradiating light, it is possible to check how much of a compound is present there.
The UV-visible absorption spectrum is a well-known measurement technique that utilizes light, and is used in many research institutions. In addition to detecting ultraviolet and visible light, there is also a widely used technique to observe fluorescence. This is the fluorescence analysis method.
Instead of measuring how much light is absorbed, fluorescence spectroscopy measures how much fluorescence is observed (how strong the fluorescence is).
Research institutions that conduct biochemical experiments, in particular, often make use of fluorescence spectroscopy. In this section, we will explain the principles of fluorescence spectroscopy and the concept of the measurement method.
Table of Contents
- 1 Conjugated Structures Can Produce Fluorescence
- 2 Phosphorescence Is a Long-Lasting, Weakly Fluorescent Phenomenon
- 3 Measuring the Degree of Fluorescence Will Give Us the Concentration of the Compound
- 4 Use of Compounds with Fluorophore in Experiments
- 5 Principles and Applications of Fluorescence Spectra in Chemistry
Conjugated Structures Can Produce Fluorescence
Chemical phenomena occur all around us. Among them, fluorescent reactions are usually familiar to us. There are many fluorescent products available, and fluorescent products are also used in events.

Fluorescence is widely used in scientific experiments as well as in products.
Organic compounds may fluoresce if they have a conjugated structure. Fluorescent substances exist in nature and in synthetic products. Examples are the following substances.
- Chlorophyll
- Coumarin
- Fluorescein
- Rhodamine
All of these substances have a conjugated structure and emit fluorescence as a result. Of course, not all molecules in the conjugated structure produce fluorescence. And even when they do, they often emit very weak fluorescence.
In fluorescence spectroscopy, we use compounds that emit strong fluorescence. This makes it possible to obtain fluorescence spectra.
After the Excited State, Fluorescence Appears As Light Energy
Why does fluorescence appear? This is because the molecules release energy as light.
In most molecules, the electrons are in a stable place (ground state). However, when light energy hits the molecule, the electrons move to an unstable orbit as a result of receiving energy from the light. This phenomenon is called an electronic transition. The unstable state is called an excited state, and electrons do not like an unstable state.
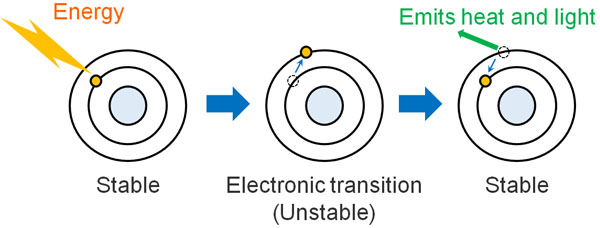
Electrons try to return to a stable state by releasing energy. They don’t stay in an excited state forever.
In most cases, when they release energy, the molecules emit it as heat. However, there are some substances that release energy as light instead of heat. For compounds that have conjugates in their structure, the probability of releasing light energy is higher.
This is the simple principle behind fluorescence when light is shone on a fluorescent substance. Fluorescence is observed when the molecule receives energy from the light and releases light energy.
Fluorescence Spectra Different from the Excitation Light Are Measured at Long Wavelengths
It is important to note that a different wavelength of light is observed as fluorescence than the light we shine (excitation light). For example, when we shine a black light (slightly visible long-wave UV light), it emits light that we can see.
This means that light of a different wavelength (fluorescence) than the excitation light is being emitted from the molecule.
As mentioned earlier, when light energy is applied, electronic transitions occur, and the molecule enters an excited state. From the excited state, the molecule returns to a stable state, but not all of it is released as light energy. Most of it is released as heat energy because that is inefficient.
Since the incoming energy and the released energy are the same, we can think of it as follows.
- Excitation light energy = Released heat energy + Released light energy (fluorescence)
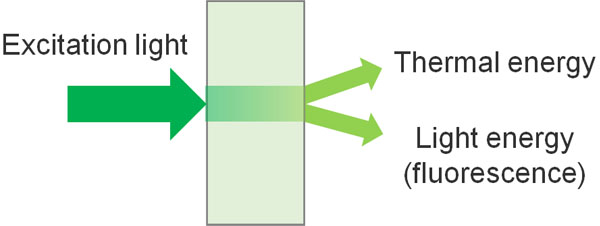
It is important to understand that the energy emitted includes heat. Therefore, when comparing the excitation light and the emitted light energy (fluorescence), we can expect the energy of fluorescence to be weaker by the amount of heat energy.
As a result, colors are observed at longer wavelengths (lower energy) in fluorescence compared to excitation light.
Black light is a type of ultraviolet light that has high energy and is difficult to see. Therefore, when a black light is shone on a fluorescent material, visible light (low-energy, long-wavelength light) can be observed.
The Measurement Method Is a Two-Peak Observation Including Excitation Spectra
In contrast to the general spectra, two peaks are observed in fluorescence spectroscopy. The first is the excitation spectrum. A fluorescent material needs to be illuminated, and the incoming light is the excitation light. The data obtained by the excitation light is the excitation spectrum.
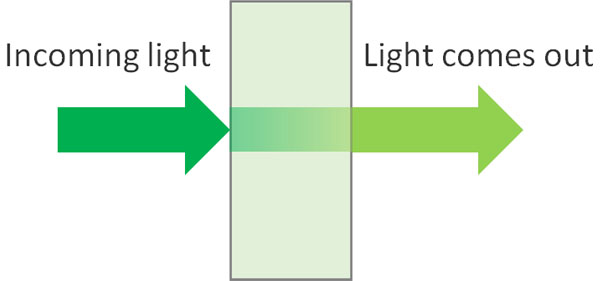
When you shine an excitation light on a molecule, the molecule absorbs the light energy. In other words, the light of the excitation light that comes out is weak. So by observing the intensity of the excitation light that comes out, we can obtain the excitation spectrum.
On the other hand, by shining the excitation light, fluorescence can be observed. We have already explained that fluorescence has a longer wavelength than the excitation light. Therefore, not only excitation spectra, but also fluorescence spectra can be obtained.
- Excitation spectra: Data obtained from the output light
- Fluorescence spectrum: data of light observed as fluorescence
The two have these differences.
For the excitation spectrum, it is sometimes called the absorption spectrum because it is obtained as a result of the absorption of light by a molecule. Strictly speaking, the excitation spectrum and the absorption spectrum are not the same. However, since they are very similar, there is little reason to understand the difference between the two, so it is important to understand that they are the same.
On the other hand, excitation spectra and fluorescence spectra are completely different. We should understand the difference between them. In any case, consider that fluorescence spectroscopy provides two spectral data, one for excitation light and one for fluorescence.
Phosphorescence Is a Long-Lasting, Weakly Fluorescent Phenomenon
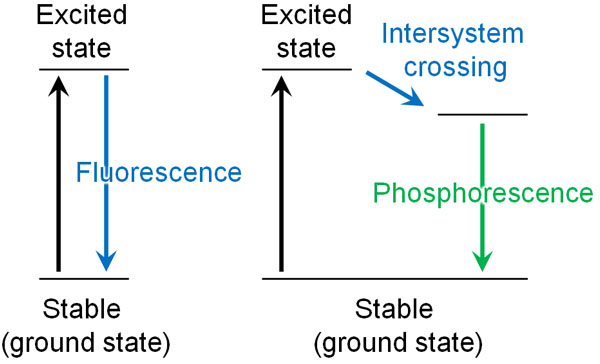
When we learn about fluorescence, we often study phosphorescence as well. Although fluorescence and phosphorescence are similar, they have different characteristics.
If you shine a black light on them, they emit fluorescence. However, if we block out the light, we cannot see the fluorescence. Fluorescence can only be observed when the excitation light is shining on it.
On the other hand, some fluorescent materials continue to emit light for a certain period of time, even after the light exposure is stopped. This is called phosphorescence. For example, the picture below shows a road that receives sunlight during the day and shines with phosphorescence at night.

In the case of fluorescence, the light stops emitting the moment it is no longer exposed to light. Phosphorescence, on the other hand, continues to emit light even when it is no longer illuminated.
Principle of Generating Phosphorescence: Intersystem Crossing and Triplet Excited State
Why does phosphorescence occur? When an excitation light strikes the material, an electronic transition occurs, and the material becomes excited. The electrons then return to the ground state (the original state). In this case, the electrons emit fluorescence.
On the other hand, in some molecules, the state of the electron spin may change after the excitation light hits the molecule. This is called intersystem crossing.
Normally, the electron spins are oriented in opposite directions to each other. This is called a singlet excited state. On the other hand, when intersystem crossing occurs, the electron spins point in the same direction. This state is called a triplet excited state.
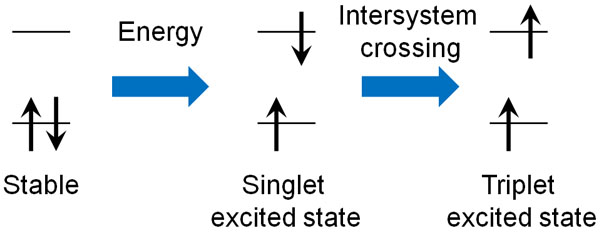
After the excitation light hits the molecule, some molecules undergo intersystem crossing, as mentioned above. After entering the triplet excited state, the electrons try to return to the lower energy ground state.
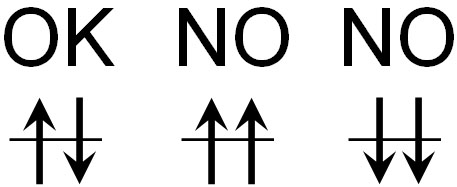
It is important to note that electrons do not enter the orbit facing the same direction. They must be paired and pointing in opposite directions to enter the orbit. This is called the Pauli exclusion principle.
Even if an electron wants to return to a stable energy state, it cannot easily do so because the electron spins are pointing in the same direction. As a result, it will emit weak light as phosphorescence for a long time.
After the light is gone, fluorescence immediately loses its light. Phosphorescence, on the other hand, continues to emit light for a long time. This difference is due to the different orientations of the electron spins.
Measuring the Degree of Fluorescence Will Give Us the Concentration of the Compound
How can fluorescence be used in scientific experiments? In this regard, it is used to measure concentration.
After exposing a fluorescent substance to light, the fluorescence can be observed. The higher the concentration of the fluorescent material in the solution, the stronger the intensity of the fluorescence observed. From these results, it is possible to measure the concentration of the substance in the solution.
By looking at the excitation spectra, we can determine which wavelengths are needed to excite the fluorescent substance. The fluorescence spectrum also tells us which wavelengths of light are most strongly emitted. By measuring this, we can determine the light to be excited and the light to be observed as fluorescence.
For example, if the fluorescent substance is colored as the reaction proceeds, then we can determine how much of the fluorescent substance is present in the solution, which allows us to learn about the molecule.
There are several drawbacks with techniques that measure the absorption of light, such as UV-visible absorption spectroscopy. One of the most significant drawbacks is that there are so many organic compounds with conjugated structures that, in most cases, they absorb UV light.
Even if absorption spectra are observed, it is not clear whether the absorption spectrum is due to the target compound or not.
On the other hand, fluorescence is a substance-specific light. Therefore, even if multiple substances are mixed in, only the specific substance can be examined by measuring the intensity of the fluorescence emitted. Although there are few substances that emit fluorescence, the use of substances that emit fluorescence increases the accuracy of scientific experiments.
The Wavelength and Intensity of the Spectrum Changes with Solvent Effects and Temperature
Other influences should be taken into account when using fluorescence spectroscopy. A compound that emits fluorescence is an inherent ability of the molecule. However, which fluorescence is emitted depends on the surrounding environment.
For example, different solvents will change the wavelength and intensity of the fluorescence spectrum. Many factors, including the length of the observed fluorescence wavelength and how strong the fluorescence is emitted, will differ. This is called the solvent effect.
Because of the solvent effect, we need to use the same solvent and standardize the conditions when we do scientific experiments for fluorescence measurements.
In addition to the solvent effect, the temperature is also a factor to be considered. Fluorescence intensity is affected by temperature. The higher the temperature, the weaker the fluorescence intensity becomes. In other words, the higher the temperature, the weaker the peak of the fluorescence spectrum, resulting in less accurate data.
Fluorescence measurements can be affected by the surrounding environment. Therefore, make sure that all conditions are the same for the measurement.
Remove Unwanted Peaks, Such As Raman Scattering (Raman Spectra)
When measuring fluorescence, it would be nice if we could measure the fluorescence spectrum of just the target compound, but this is not always the case. Other unwanted peaks may also be observed.
For example, one of the things we observe when measuring fluorescence spectra is that we get a Raman spectrum. Raman scattering is a type of light scattering that occurs when a substance is illuminated. When Raman scattering is observed, Raman spectra can be obtained.
In other cases, fluorescence peaks caused by solvents can also be observed.
However, in the case of fluorescence spectroscopy, what we want to study is not the Raman scattering or the light peak caused by the solvent. Therefore, we measure the fluorescence spectrum using only the solvent. After that, the fluorescence spectrum of the target compound is obtained by subtracting the observed spectrum of the solvent.
By omitting these unwanted peaks, we obtain the following clean peaks.
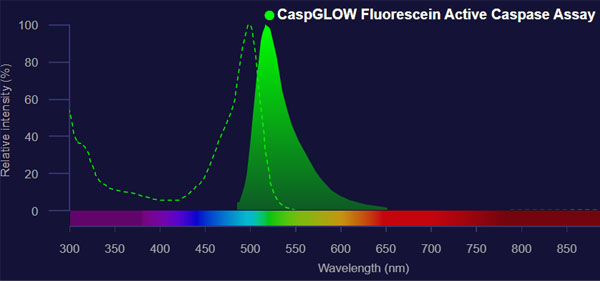
Source: Molecular Probes
This data contains both excitation and fluorescence spectra. In fluorescence spectroscopy, both excitation and fluorescence spectra are always present.
If you do not operate anything, it is common that other peaks such as Raman spectra and solvent are mixed in. Therefore, it is common to measure the solvent alone as a blank.
Use of Compounds with Fluorophore in Experiments
Fluorescent materials are often used in scientific experiments. However, compounds are rarely fluorescent. Substances that emit fluorescence are limited, and the number of compounds is small.
Therefore, when fluorescence is used in biochemistry and other experiments, compounds with a fluorophore are used. As mentioned above, chlorophyll, coumarin, fluorescein, and rhodamine are known as substances that emit fluorescence (fluorochromes).
For example, we attach a fluorophore to an antibody. Since antibodies only bind to specific substances, it is possible to visualize where antibodies are clustered when they are introduced into a cell.
Many experiments in biochemistry have been carried out using fluorescence, as shown below.
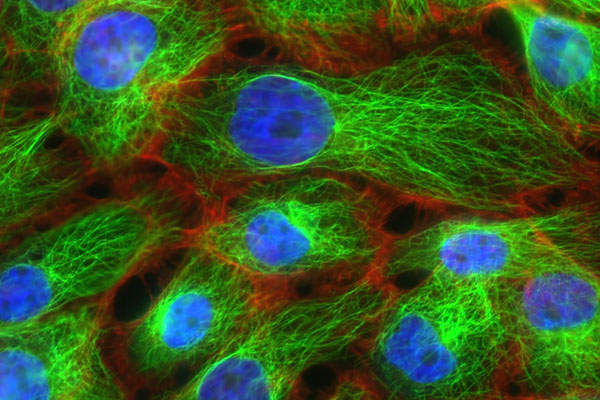
The picture above is not a method of measuring the concentration of a compound by fluorescence analysis. However, you can use fluorescent substances to understand the movement of molecules.
You can also bind reagents with a fluorophore. The reaction between the target compound and the reagent causes it to fluoresce. By measuring the intensity of the fluorescence, you can measure the concentration of how much of the target compound is in the solution.
Other common uses of fluorescence spectroscopy are in mineral identification. Fluorescence can be used to distinguish if a gemstone is real or not. The intensity of the fluorescence can also be used to determine the grade of a gemstone.
Xenon lamps are the most common light sources used in fluorescence spectroscopy. By shining such a light, the fluorescence intensity is measured.
The 2008 Nobel Prize in Chemistry Was Awarded for Research on GPF (Green Fluorescent Protein)
For reference, the winner of the 2008 Nobel Prize in Chemistry had researched fluorescence. They won the Nobel Prize for their work on a green fluorescent protein called GFP.
GPF exists as a protein, and GPF emits fluorescence. So by using fluorescence, we can see where the fluorescent protein is located in the cell. Fluorescent proteins are indispensable for molecular imaging (technology to visualize where molecules are located).
By measuring the intensity of fluorescence, it is also possible to confirm how many target molecules are present in a cell.
GFP is used in many fields, such as genetic recombination technology, molecular imaging, and the visualization of molecular movements.
Fluorescent materials are one of the most important substances in the field of science and technology. A clear example is GFP (green fluorescent protein), which was awarded the Nobel Prize in Chemistry. Fluorescence is very important in the field of biochemistry.
Principles and Applications of Fluorescence Spectra in Chemistry
One very important discipline for any chemistry major, including biochemistry and organic chemistry, is fluorescence. By observing the fluorescence spectrum, we can deduce a great deal about it.
In the past, the Nobel Prize in Chemistry was awarded for the study of fluorescence, including GFP (green fluorescent protein), which has become indispensable in molecular imaging.
Measuring the intensity of fluorescence by fluorescence spectroscopy may not seem like an important subject. However, there are many research institutions that use fluorescence in their experiments. The use of fluorescence allows us to recognize where the target compound is located. Also, by measuring the fluorescence intensity, we can determine the concentration.
Among analytical methods, fluorescence spectroscopy is one of the most important ones, as well as UV-visible absorption spectroscopy. Make sure you understand the principles and applications of fluorescence.