NMR (Nuclear Magnetic Resonance) is important when determining the structure of a compound. By studying the environment of the protons (hydrogen), you will be able to guess the structural formula.
However, when you use NMR, one thing that requires special knowledge is the benzene ring. If you don’t understand the benzene ring and how it couples together, you won’t be able to guess the structural formula when you look at the NMR peak.
What’s particularly important about the benzene ring is what happens in the environment in the ortho and meta positions. This will lead to different chemical shifts. At the same time, the substituents in the benzene ring are also important.
In NMR, we can determine the structure of the benzene ring by understanding what happens to the chemical shift. We will now explain how to understand 1H-NMR at the benzene ring.
Table of Contents
Different Peak Coupling in Ortho and Meta Positions
In alkyl chain coupling, you only need to focus on the hydrogen next to it. The coupling is influenced by the neighboring hydrogen. As a result, the peak splits as shown below.
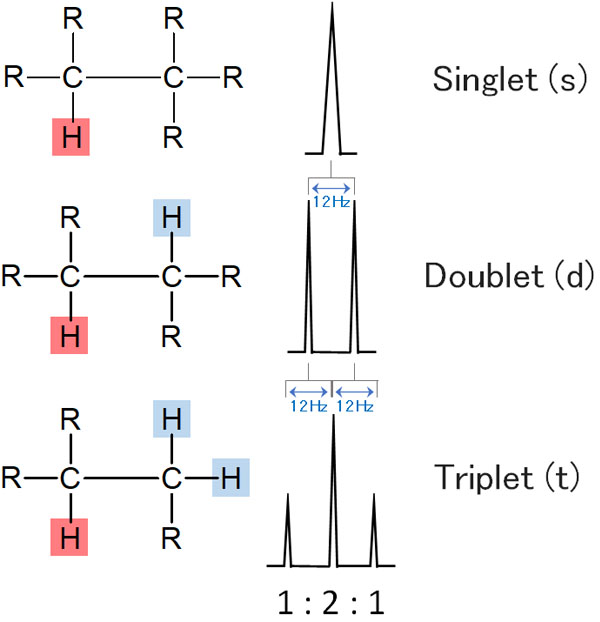
The same thing happens in the case of a benzene ring. But in the case of a benzene ring, it is affected by the hydrogen in the ortho position, and even by the hydrogen in the meta position. Therefore, a slightly more complicated peak appears.
The hydrogen at the para-position of the benzene ring is unaffected by coupling. So we can ignore it, but we need to understand how the proton (hydrogen) shows up in the ortho and meta positions.
Ortho-Couplings Have a High J-Value
The closer the proton is to the other hydrogen atoms, the greater the effect on the proton. For the ortho position of the benzene ring, the J-value will be higher if a hydrogen atom is present.
This is called ortho coupling, and it couples with a J-value of about 6 to 9 Hz.
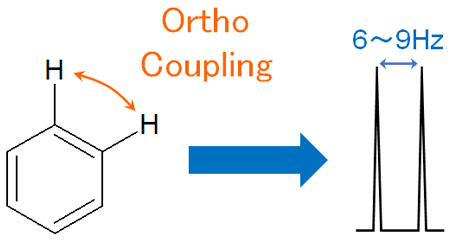
It is easy to predict that the closer the distance is, the greater the interaction and the greater the J-value.
Meta-Coupling Has a Low J-Value
On the other hand, the benzene ring is affected by the hydrogen atom in the meta-position. We need to check not only the hydrogen atom bonded to the neighboring carbon atom, but also other hydrogen atoms. Therefore, it is important to pay attention to the meta-position in the NMR (Nuclear Magnetic Resonance) of the benzene ring.
This is called meta-coupling, and when the meta-position of the benzene ring contains a hydrogen atom, the J-value is usually between 1 and 3 Hz.
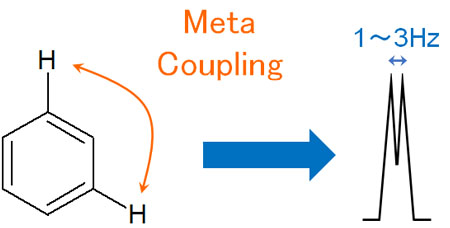
Compared to the ortho position, the J-value is lower due to the distance between the two positions in a meta-coupling.
Check Benzene Ring coupling for Each Case
How are the peaks observed when a compound with a benzene ring is actually measured by NMR? Since it is impossible to understand without considering actual examples, we will present several examples.
They are as follows.
Benzene Ring with Only Ortho Coupling
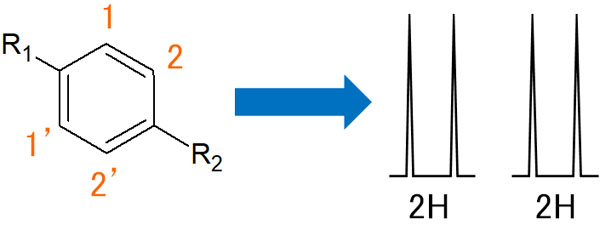
In the case of this compound, it will only do ortho-coupling. It will only interact and couple with the neighboring hydrogen atoms. As a result, two doublets will appear.
Some people might think that the 1 and 1′ would interact and couple in the meta-position. However, the 1 and 1′ hydrogens have completely the same properties. Since they do not interact with each other, no meta-coupling occurs as a result.
Note that the proton of the benzene ring observed by NMR is 2H (equivalent to two hydrogen atoms). Since there are two hydrogen atoms of the same nature, it is important to understand that the integral value of two protons is measured.
Coupling with a Benzene Ring with Three Substituents
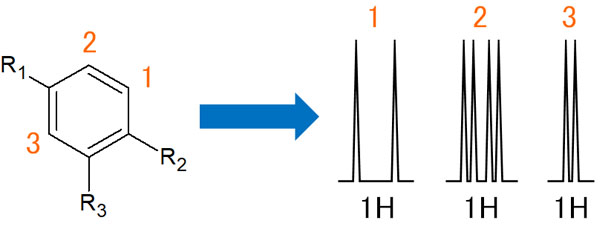
Next, consider a benzene ring with three substituents in the arrangement shown in the figure above.
From position 1, only the number 2 proton interacts. The result is a doublet by ortho-coupling.
On the other hand, what about the hydrogen atoms of 2? First, they are affected by the proton of 1. In other words, it produces ortho coupling. Furthermore, it is also affected by the hydrogen atom in position 3, resulting in meta-coupling. The result is as follows.
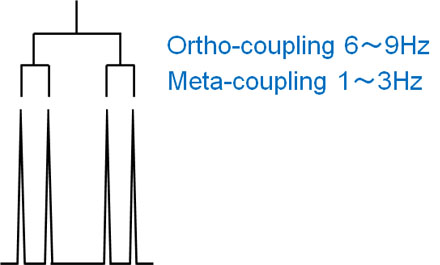
When both ortho and meta-coupling are considered, the spectrum appears as quadruple lines.
Coupling of Protons with Meta-Substituents
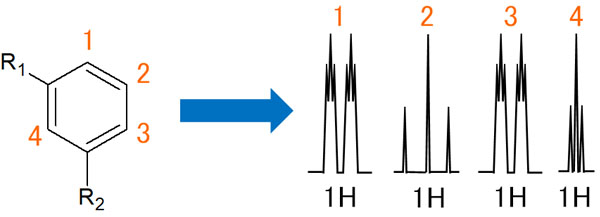
We will check the case of the benzene ring in the above figure, which has a substituent at the meta-position.
In terms of the 1 proton, it is ortho-coupled at the 2 hydrogen atom. Therefore, it becomes a doublet. Also, because of the meta-coupling with the proton of 3 and 4, a small triplet is observed for each doublet.
On the other hand, only the protons of 1 and 3 interact with the hydrogen atoms of 2. The ortho-coupling causes a triplet to appear.
In the case of the 3 hydrogen atoms, the ortho coupling with the 2 protons results in a doublet. Afterwards, it is meta-coupled with 1 and 4 hydrogen atoms to form a small triplet.
Also, the 4 hydrogen atoms will meta-couple with the 1 and 3 hydrogen atoms, resulting in a very small triplet. As a result, a very small triplet appears.
Coupling with Ortho-Substituents
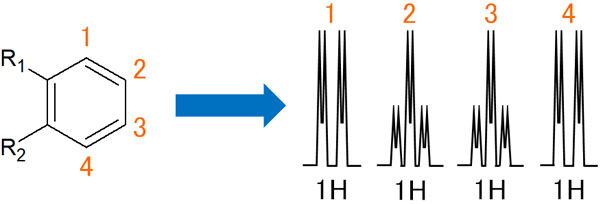
Suppose that there is a benzene ring with a substituent at each ortho position. What will the peak spectrum of NMR (Nuclear Magnetic Resonance) look like in this case?
At the 1 hydrogen, there is ortho coupling with the number 2 proton. In other words, it becomes a doublet. Then it is meta-coupled with the proton of 3. As a result, the doublet splits into two more. The same phenomenon occurs for the hydrogen atom in 4.
On the other hand, what about the proton 2, which is ortho-coupled with the hydrogen atoms of 1 and 3 to form a triplet. It then meta-couples with the hydrogen atom of 4, and each peak splits in two. The spectrum of the 3 hydrogen atom is the same as the peak of the 2 hydrogen atom.
-If the Substituents Are the Same, the Peaks Will Overlap
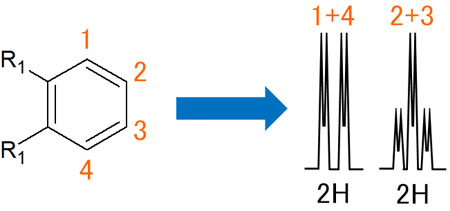
If the substituents in the ortho position are the same, then the hydrogen atoms in 1 and 4, 2 and 3 have the same properties. In other words, they can be considered to be completely identical. As a result, the observed spectral peaks will overlap.
Depending on whether the substituents are the same or not, the observed spectra will be different.
Spectra of Benzene Rings with a Single Substituent
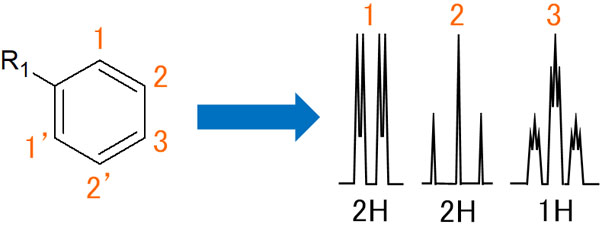
On the other hand, what does the NMR peak look like in a benzene ring with only one substituent?
First, let’s focus on the hydrogen atom at number 1. It is ortho-coupled with the proton at number 2 to form a doublet. It then interacts with the hydrogen atom of 3 and the peak is further split into two.
As a reminder, 1 and 1′ are the same hydrogen. Therefore, there is no meta-coupling. Also, due to the same nature of hydrogen, the peaks overlap and are observed in the integral ratio of two hydrogen atoms. We can understand that hydrogen atoms of the same nature do not interact with each other.
On the other hand, what about the hydrogen atom of 2. The proton of 2 is ortho-coupled with the hydrogen atoms of 1 and 3. Therefore, a triplet is observed, and since the 2′ is a hydrogen of the same nature, it cannot be meta-coupled.
In addition, a 3 hydrogen atom will be ortho-coupled with the 2 and 2′ hydrogen atoms. Therefore, it is a triplet. After that, it will meta-couple with the 1 and 1′ hydrogen atoms. As a result, each peak is a small triplet.
Chemical Shifts in Low or High Magnetic Fields Are Inferred by Substituents
With this in mind, let’s take a look at the NMR spectrum of the benzene ring. The next thing to consider is what happens to the NMR chemical shifts (where the peaks appear). In other words, we need to understand where the peaks are observed in low or high magnetic fields.
Even within the same benzene ring, the electron density differs depending on the substituents. So make sure you learn in advance how the substituents affect it.
You should understand the following
- Electron-donating groups shift to high magnetic fields: amino groups, hydroxy groups, etc.
- Electron-withdrawing groups shift to low magnetic fields: nitro groups, etc.
Although the concepts and principles are not difficult, an understanding of chemical shifts is essential in order to understand which hydrogen atoms are the peaks that appear in NMR.
Electron-Donating Groups Such As Amino and Hydroxy Groups Are Shifted to Higher Fields
When the electron density is high, the peaks are not observed unless more energy is given to them. Substituents in the benzene ring, such as amino groups (-NH2) and hydroxy groups (-OH), are known to be electron-donating groups.
When there is a nitrogen or oxygen atom in the alkyl chain, electrons are pulled due to electronegativity, resulting in a lower electron density. In other words, the NMR chemical shift is low-field shifted. The peaks are observed at lower fields.
On the other hand, if an amino or hydroxy group is attached to the benzene ring, these substituents become electron-donating groups. This means that the electron density of the benzene ring is high. As a result, the hydrogen atoms affected by the electron-donating groups show peaks in high magnetic fields.
- -NH2
- -OH
- -NR2
- -OCH3
For example, these substituents are known to be typical electron-donating groups. Of course, alkyl chains, such as methyl groups, are also electron-donating groups.
Electron-Withdrawing Groups, Such As Nitro Groups, Have a Low Magnetic Field Shift
On the other hand, there are also electron-withdrawing groups. They are substituents that reduce the electron density of the benzene ring by attracting the electrons of the benzene ring. These electron-withdrawing groups are known to be the following.
- -COOH
- -C≡N
- -CHO
- -COOCH3
- -NO2
- -Cl
As a result of these effects on protons, the chemical shifts in NMR show a peak at low magnetic fields. For example, suppose that the following compounds are present.
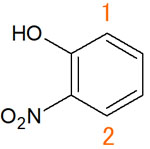
At the hydrogen atom of 1, there is an electron-donating group (-OH) next to it. Therefore, the chemical shift is considered to be a high field shift. On the other hand, a 2 hydrogen atom has an electron-withdrawing group (-NO2) next to it. This means that the proton of 2 peaks at lower magnetic fields.
Atoms of 1 and 2 have the same shape. However, the position of the chemical shift is different. Although the peaks appear at different positions, it is possible to deduce which peak is which hydrogen atom from the position of the substituent group.
The only way to learn the electron donor and electron-withdrawing groups is to memorize them. So once you understand the nature of the substituents, you will be able to predict where the NMR chemical shifts will appear and attribute them to each peak.
Understanding the Principles of 1H-NMR Spectra and Attributing Peaks
The most frequently used NMR is 1H-NMR, so let’s first understand only 1H-NMR.
For the coupling of the alkyl chain, we can consider the effect of the hydrogen atoms that bind to the neighboring carbons. However, in the benzene ring, it is not only affected by the hydrogen atom in the ortho position, but also by the hydrogen atom in the meta position. So let’s understand how the NMR peaks show up.
The chemical shifts caused by substituents are also important. We have to guess where the peaks appear in low or high magnetic fields.
If we can predict the shape of the peaks and the location of the chemical shifts, we can attribute them to each peak. This also makes it possible to determine the structure of the compound. The NMR of the benzene ring is a bit unique, so it is important to understand its characteristics.