When we want to measure the state of a substance, we have to analyze it. There are various methods of analysis, and one of them is Raman spectroscopy.
Molecules vibrate with each other. By examining the degree of vibration between these molecules, we can check what happens to the compound. We shine a light on them and see what happens to the light (spectrum) that comes out.
The peaks observed by Raman spectroscopy are called Raman spectra. Raman spectra are used in the structural analysis of compounds, and are used to measure known substances.
So what is the principle behind Raman spectra? We will discuss the concept of Raman spectra.
Table of Contents
- 1 Used for Comparison with Known Substances, Not for Structure Determination
- 2 Ordinary Scattering Is Rayleigh Scattering
- 3 Rayleigh Scattering Occurs in Electron Clouds Due to Dipole Moments
- 4 What Is the Difference Between the IR Spectrum and the Raman Spectrum?
- 5 Understand the Principles of Measurement and Use the Raman Spectra
Used for Comparison with Known Substances, Not for Structure Determination
Complementing the data from infrared spectroscopy is the Raman spectrum. The IR spectra obtained by the IR absorption method are shown below.
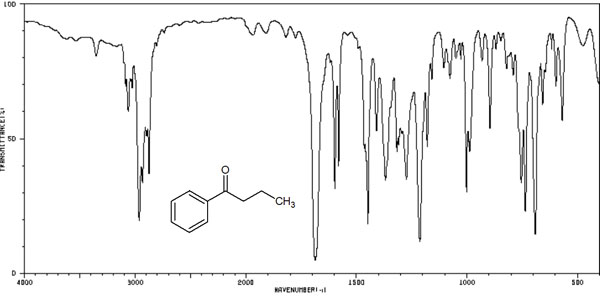
The Raman spectrum provides similar data to this.
If you look at the IR spectrum (infrared absorption spectrum), there is very little data to read from it. In the same way, if you try Raman spectroscopy, there is very little information you can get from that data. Even if you measure an unknown compound, you will not know anything from it.
On the other hand, it is useful when examining a known substance because the spectral data shows a large number of complex peaks. If the shape of the spectrum is the same as that of the reference substance, then we can assume that they are the same substance.
Think of both IR and Raman spectra as being used in structural analysis to study a known substance.
Raman Spectroscopy to Measure the Energy Difference in Raman Scattering
What does Raman spectroscopy investigate? In Raman spectroscopy, we measure the energy of scattered light.
When light hits a molecule, it is either transmitted, absorbed, or scattered. Many analytical techniques, such as UV-visible absorption spectroscopy and infrared absorption, measure the absorption of light. When light is irradiated, they measure how much the light that comes out is weakened.
On the other hand, it is important to understand that Raman spectroscopy is a technique that measures how much light is scattered.
When we shine a light on a substance, a phenomenon called Raman scattering occurs. We can measure the light energy generated by Raman scattering and observe the peaks in the spectrum.
Ordinary Scattering Is Rayleigh Scattering
The most common phenomenon of light scattering is Rayleigh scattering. The refraction of light is Rayleigh scattering. Every day we see this phenomenon in Rayleigh scattering.
For example, why is the sky blue? And why is the sunset red? All of these phenomena are caused by Rayleigh scattering.

When light hits a molecule, it is scattered in various directions. Light at red wavelengths is less likely to scatter, and light at blue wavelengths is more likely to scatter. As a result, in the evening, the blue light is scattered before it reaches our eyes, leaving a red color. As a result, the sunset is red.
We see this phenomenon caused by Rayleigh scattering all the time. From this fact, we can understand that light is scattered when it hits a substance.
Observe Stokes Scattering or Anti-Stokes Scattering
However, Raman scattering is not the same as Rayleigh scattering. Normally, the scattering of light is Rayleigh scattering, but it is important to understand that Raman scattering is a special type of scattering.
When external light energy is applied to a molecule, the electrons in the molecule gain higher energy. As a result, an electronic transition occurs. This is called an excited state, and it is an unstable state.
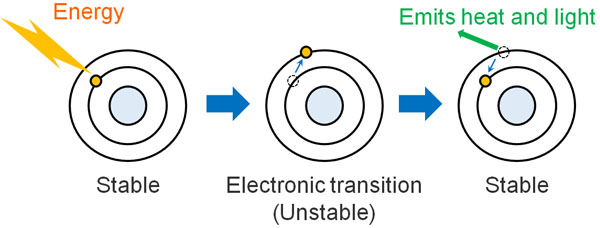
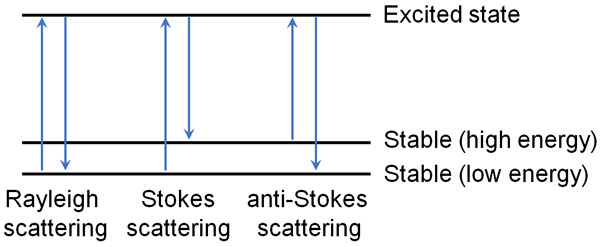
But the substance does not like instability. It tries to return to its original stable state (ground state) by releasing energy. This is what happens in Rayleigh scattering.
However, in some cases, after the addition of light energy, the light can become more energetic than in its original state. It absorbs energy from the light and scatters it. This is called Stokes scattering.
On the other hand, after adding light energy, it can settle to lower energy than the original state. After hitting the light, light absorbs the vibrational energy of the molecule, causing it to lower its energy. The scattering observed in this case is called anti-Stokes scattering.
Even the same substance has different energies when it is in motion. For example, the bonds between atoms are like springs, which vibrate as follows.
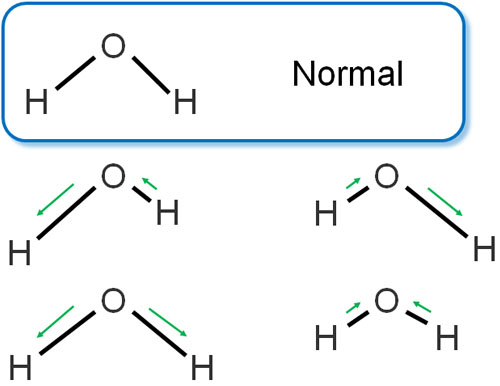
Naturally, different states of vibration have different energy states. Even the same substance has different energies. After the light hits the molecule, it returns to its original state (ground state), but it may vibrate differently. As a result, they have different energies.
In the case of Rayleigh scattering, the energy of the light doesn’t change, which means that nothing can be observed.
On the other hand, if Stokes or anti-Stokes scattering occurs, the light gives energy to the molecules (or reduces their energy). This means that after scattering, the intensity of the light changes, and the color of the light also changes.
Shining light on a molecule causes a change in the energy and color of the light that comes out. If the energy of the light changes, the vibrational energy (frequency) of the molecule changes by the same amount. These phenomena are known as Raman scattering.
Rayleigh Scattering Occurs in Electron Clouds Due to Dipole Moments
So why does adding externally light energy cause a difference in energy? Let’s start with the reason for Rayleigh scattering.
Electrons have an electric charge. If there is no electric field (the space that affects the electrons), no reaction occurs.
On the other hand, what if there is an electric field? If there is a positive or negative electric field, the electron cloud in the molecule will have a different charge. They will be separated into positive and negative charges within the molecule, respectively. This is called the dipole moment.
Light has waves, and light is a type of electromagnetic wave. In other words, light has an electric field. When light with an electric field hits a substance, the molecule is divided into positive and negative charges and has a dipole moment.
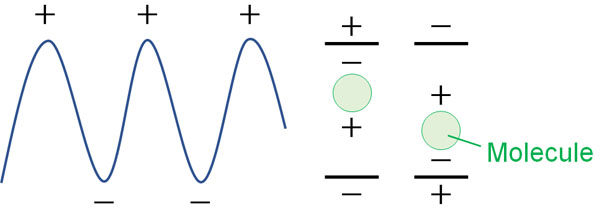
The dipole moment is induced by the electric field, and this is called the induced dipole moment.
Since light is a wave, it is always vibrating. The induced dipole moment vibrates in response to the vibration of light. In other words, the molecules move. As a result, we observe scattered light with the same frequency as the entering light. This is Rayleigh scattering.
Focus on the Atoms and Raman Scattering Occurs
Next, let’s look at atoms. The same is true of atoms: they have a charge. The nucleus of an atom has protons and neutrons. In other words, the atom is vibrating.
So when we shine light energy on an atom, the electric field caused by the light creates a dipole moment. But not only that, the vibrations of the nucleus will also produce differences in energy.
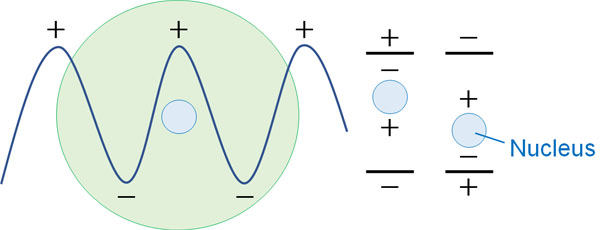
Compared to the movement of electrons in molecules, the vibrations of atomic nuclei are small. Therefore, the energy difference is small. However, it still makes a difference in the observed energy.
The interaction between the vibrational frequency of the light and the vibrational frequency of the atoms causes a difference in the energy after scattering. This is Raman scattering, which is observed as a Raman spectrum.
Raman Scattering Is Weak Light, So a Laser Is Used
Compared to the movement of electrons, the difference in energy is small because it measures the difference in the vibration of atoms. Also, most of the scattered light is Rayleigh scattering, and there are few cases that Stokes scattering or anti-Stokes scattering is caused by Raman scattering.
In other words, Raman scattering light is very weak. Since it is mostly Rayleigh scattering, it is difficult to observe Raman scattering with ordinary light.
Therefore, a laser is used. We are able to observe Raman scattering by shining an extremely strong light, such as a laser. Even if the observed energy is weak, the peak of the Raman spectrum can be easily observed by increasing the output energy.
What Is the Difference Between the IR Spectrum and the Raman Spectrum?
In the Raman spectrum, it is often compared to infrared spectroscopy. What is the difference between the Raman spectrum and the IR spectrum?
The unit of measurement for both Raman and infrared spectroscopy is cm-1. However, with Raman spectroscopy, the type of vibration that can be observed is different. When a molecule undergoes stretching vibrations, there are two types of vibrations that can be observed.
-Two Bonds Stretch and Contract at the Same Time
![]()
-Two Bonds Are Alternately Stretched and Contracted
![]()
The simultaneous stretching and contracting of the bonds cannot be observed in the IR spectrum. On the other hand, it can be measured by Raman spectra.
Then, what about the case of alternating stretching and contracting of bonds? This can be measured by infrared spectroscopy. However, this cannot be measured by Raman spectroscopy.
There are some things that cannot be determined by infrared spectroscopy that can be determined by Raman spectroscopy. And often, the opposite is true. The reason why Raman spectra can be used as a support for infrared absorption spectra is that they can compensate for each other’s inability to measure.
Understand the Principles of Measurement and Use the Raman Spectra
Based on these principles, Raman spectroscopy is used to observe the vibrational spectra of molecules. The peaks in the Raman spectrum are observed as light scattering rather than absorption of light energy.
The Stokes and anti-Stokes scattering peaks observed in Raman spectra are substance-specific. If the compound is different, the observed Raman spectra are also different.
Although it is impossible to determine the structure of an unknown compound, it is possible to determine whether a compound is a target compound or not by comparing it with a known compound. Raman spectroscopy can measure different peaks than infrared spectroscopy (infrared absorption spectroscopy) because it uses different principles.
It is important to understand these principles before using Raman spectra.





