In a laboratory that mainly focuses on the synthesis and separation of organic compounds, thin-layer chromatography (TLC) is an analytical technique that is used every day.
Typically, chromatography is used to separate substances. Of course, thin-layer chromatography can also separate compounds.
However, rather than separating a large number of compounds that have been synthesized or extracted, thin-layer chromatography (TLC) is used to determine if the synthetic reaction of a compound has been completed. Alternatively, it is used to see how many compounds are mixed in a solution.
You can’t use thin-layer chromatography without understanding the principles. So we will explain the principles and features, how to read the Rf value, and even how to choose a developing solvent.
Table of Contents
Adsorb Silica Gel (Stationary Phase) onto a Glass Plate
Among the analytical methods, TLC is a glass plate with silica gel adsorbed on it. The following thin plate is TLC, which is used in chemical experiments.

Thin-layer chromatography is not limited to silica gel, but also uses alumina. But not alumina, silica gel is the most common. Unless there is a special reason, we use silica gel TLC, not alumina.
Thin-layer chromatography is a type of adsorption chromatography. In adsorption chromatography, there is always a stationary phase (the immobile solid part). In thin-layer chromatography, silica gel is the stationary phase.
The structural formula of silica gel is as follows.
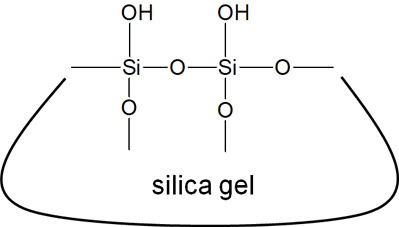
Due to the high number of oxygen atoms in the structure, silica gel is a very polar material. This property is used to check if the compound has been synthesized correctly.
What Makes It Different from Other Types of Chromatography?
What makes thin-layer chromatography different from other types of chromatography? In chromatography, there are other types of chromatography that exist, such as
- Column chromatography
- HPLC (high-performance liquid chromatography)
- Paper chromatography
As mentioned above, thin-layer chromatography is not an experimental procedure used to completely separate substances. Thin-layer chromatography is an analytical technique used to determine the progress of organic synthesis reactions and the number of compounds present in the solution.
On the other hand, column chromatography and high performance liquid chromatography (HPLC) can be used to separate substances. Column chromatography is used to achieve complete separation of compounds, while HPLC not only separates compounds, but also provides detection and concentration measurements.
Compared to these analytical methods, thin-layer chromatography can only tell us how far the organic synthesis reaction has progressed and how many compounds are present in the solution.
With paper chromatography, the stationary phase is paper instead of a thin plate of silica gel. This makes it a very simple method that can be used in elementary school science experiments. We don’t use paper chromatography in university research, but it can be used in children’s scientific research.
Different Speeds of Silica Gel Stationary Phase at High or Low Polarity
So why do we use silica gel for the stationary phase? As I mentioned earlier, silica gel is a very polar material.
Water has a high polarity. On the other hand, oil (lipids) has a low polarity. As a result, water and lipids do not mix with each other.
Consider this in the same way. Silica gel is a stationary phase with high polarity. In other words, it has a high affinity for compounds with high polarity. As a result, the more polar compounds will interact with (and adsorb onto) the silica gel.
On the other hand, highly fat-soluble compounds are less likely to interact with silica gel. They are less affected by silica gel.
A solution with two or more compounds present is poured over a silica gel. Then, fewer polar compounds (high fat-solubility compounds) are less affected by silica gel and have a higher transfer rate. On the other hand, the more polar compounds are adsorbed by the silica gel and move forward little by little.

Different compounds have different polarities. The different speeds at which the compounds advance will allow you to see how the compounds are in solution.
Consideration of Organic Solvents Used in the Mobile Phase
The next thing you have to consider in thin-layer chromatography is the mobile phase. You have to consider what kind of organic solvent to use.
Thin-layer chromatography first spots a solution on the TLC. This is then immersed in the mobile phase (developing solvent). Then the solvent rises. As the solvent rises, the compound moves up. However, different compounds move at different rates, so the compounds are separated.
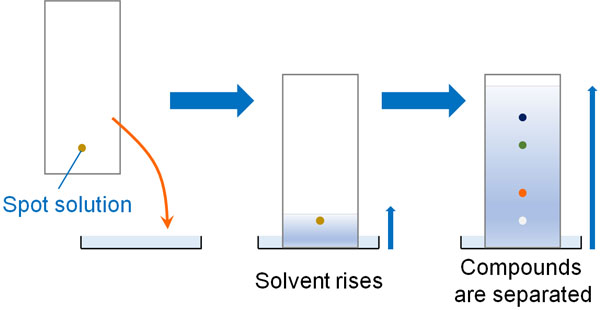
However, there are a number of different types of organic solvents. The most common developing solvent is ethyl acetate and hexane. Typically, these two organic solvents are mixed together to create the mobile phase (developing solvent) used in TLC.
Ethyl acetate and hexane each have the following structural formula.
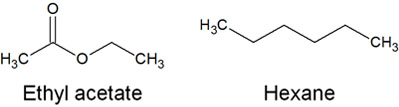
As you can see from the structural formula, ethyl acetate is highly polar. Therefore, when the ratio of ethyl acetate is high, organic compounds will dissolve out of the silica gel into ethyl acetate. In other words, it is easier to move forward with the mobile phase.
On the other hand, hexane has very high hydrophobicity, and few compounds are dissolved in hexane. Therefore, in a developing solvent with a high percentage of hexane, the compound moves forward at a slower rate.
There are many options for the ratio of ethyl acetate to hexane, such as 1:1 or 1:3. But different compounds have different properties, and no one knows which ratio is best. Therefore, it is necessary to perform TLC many times to find the best ratio.
The Rf Value Represents the Moving Distance of the Compound
When you decide on a developing solvent and do thin-layer chromatography, you get the Rf value. Rf value means the distance that the compound has moved. The Rf value can be easily obtained by calculating the distance between the developing solvent and the distance the compound moved.
- Rf value = distance moved by the compound ÷ distance moved by the developing solvent
For example, in the following conditions, the Rf values are 0.6 and 0.2, respectively.
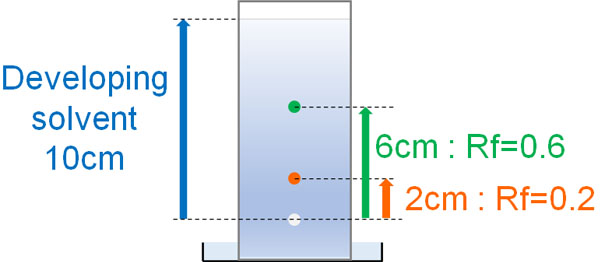
The more polar a compound is, the more it interacts with the silica gel. Since it is strongly adsorbed by silica gel, it is difficult to move forward, and the Rf value decreases. On the other hand, highly hydrophobic compounds are less likely to be adsorbed by silica gel, so their Rf value is higher.
Of course, the Rf value is completely different depending on the developing solvent. The higher the ratio of ethyl acetate in the mobile phase, the more quickly the compound moves forward with the ethyl acetate.
Make Three or More Spots in the TLC
Note that when you use TLC in an actual experiment, you need to make three or more spots. There is no case of hitting only one spot.
Why is three spots required? This is because otherwise it is impossible to determine whether the organic synthesis reaction is progressing or not. Thin-layer chromatography can also determine if the spots observed are raw materials.
In scientific experiments, it is a general rule to prepare reference material. In thin-layer chromatography, the reference material is the raw material. For example, let’s consider the following organic synthesis.
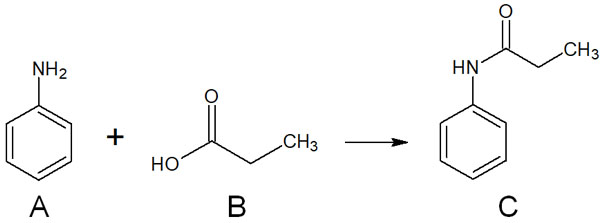
When synthesizing a compound, even if you use thin-layer chromatography to detect a spot of a compound, you cannot determine whether the spot that appears is raw material A or compound C. Since there is no reference material, you cannot determine whether the reaction is in progress, has failed, or has been completed.
So, spot the material A, material A + synthesized solution, and synthesized solution. It looks like the following.
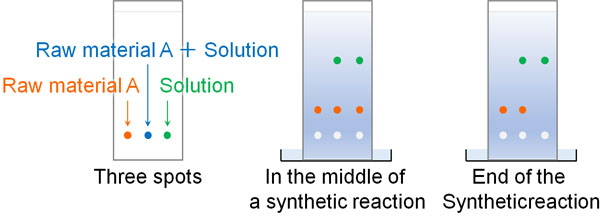
If a spot is observed at the same position as raw material A, it can be determined that the spot is raw material A. If raw material A is observed in the synthesis solution with the rightmost spot, it means that raw material A is still in the solution and the reaction is in progress.
On the other hand, if raw material A disappears, the synthesis is complete and you can confirm that you have synthesized the target compound by TLC.
There is a reason for not spotting raw material B. It is because it is not easily observable. In thin-layer chromatography, we mainly use a UV lamp to check for the presence of spots. Since raw material B cannot be checked by a UV lamp, it is not spotted on the TLC.
Determine the Developing Solvent (Mobile Phase)
After hitting the 3-point spot on the TLC, you need to determine the developing solvent (mobile phase). As mentioned above, the organic solvent to be used is mainly a mixture of ethyl acetate and hexane.
The higher the ratio of ethyl acetate, the higher the Rf value. The compound is more likely to move forward. On the other hand, as the ratio of hexane is increased, the Rf value becomes lower. As for the ratio of ethyl acetate to hexane, rely on your intuition and actually perform thin-layer chromatography many times to find the best developing solvent.
However, ethyl acetate and hexane are not the only developing solvents for TLC. The following combinations also exist.
- Dichloromethane and methanol: used for compounds with high polarity.
- Ethyl acetate and methanol: used for very polar compounds.
- Hexane and dichloromethane: used for very low polarity compounds.
There are other solvents, but these are the basic solvents to be used.
In general, you can make TLC mobile phase with ethyl acetate and hexane. However, there are rare cases when it is not possible to use these developing solvents. In such cases, a combination of other organic solvents is used to create the developing solvent.
Look for Conjugated Compounds Under UV Light (UV Lamp)
However, it is impossible to see organic compounds with the naked eye, even if you hit a spot of an organic compound. After the spotting, the organic solvent evaporates, and the plate becomes white. In such a case, how can we check the organic compounds in thin-layer chromatography?
This is done by exposing the UV light. By exposing it to a UV lamp, we can identify the organic compounds.
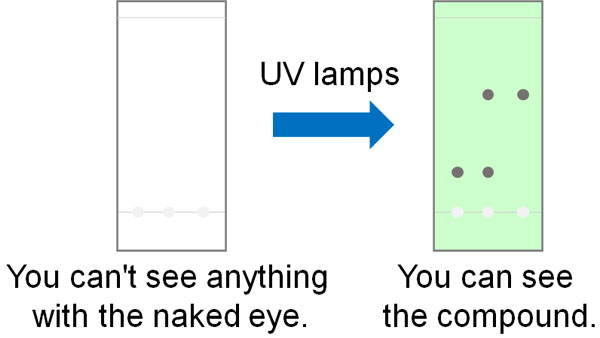
Conjugated compounds absorb UV light. For example, any compound with a benzene ring in its structural formula will absorb UV light and fluoresce. By UV irradiation with a UV lamp, compounds with conjugation in the structural formula, including benzene rings, can be confirmed by TLC.
Earlier, we explained that raw material B (propionic acid) is difficult to observe by thin-layer chromatography. This is because propionic acid does not have a conjugated structure and therefore does not fluoresce on TLC even when a UV lamp is used.
-If There Are Impurities, It Will Be Multi-Spot.
In actual synthetic research, only the target compound is rarely produced. Other compounds are often synthesized together as impurities.
It is common for multiple spots to be detected when exposed to UV light. In such cases, the target compound or impurity is determined by the light or dark color of the spots.

Or, if there are several dark spots, each compound needs to be separated by column chromatography to find out which one is the target compound.
Other Methods Such As Ninhydrin Reaction and Baking Are also Possible
However, not all compounds have a conjugated structure. There are many compounds that do not have a conjugated structure. There are also cases of organic synthesis reactions between compounds that do not have conjugated structures. In such cases, the compounds cannot be identified by UV light.
So other methods can be tried. For example, the ninhydrin reaction is known to work. If the compound has an amino acid, the color of the compound will change when the TLC is immersed in ninhydrin. In the ninhydrin reaction, the compound with the amino acid turns purple.
Another option is to bake the TLC in the oven for a few minutes. This is because it is an organic compound that often turns black when baked. If the compound evaporates due to heat, this is not possible, but if not, baking will allow you to see the compound.
When checking the results of thin-layer chromatography, sometimes the compound may not have a dye or may not fluoresce under UV light. In such cases, it is advisable to use other methods to confirm the results.
Using TLC in Organic Chemistry Experiments
Among the analytical methods, thin-layer chromatography is used every day when researching organic chemistry; TLC allows us to see if a synthetic reaction is in progress. It also allows you to check the number of compounds in the solution.
TLC is an essential analytical method when dealing with organic compounds, so be sure to understand the principle. Silica gel is used as the stationary phase (adsorbent), and highly polar compounds are adsorbed by the silica gel as they move forward.
The advantage is that it is a very simple operation and can be performed many times. However, TLC is used to check the progress of the organic synthesis reaction. You also need to consider what to use as the mobile phase and the developing solvent, and there are many organic compounds that cannot be identified by UV irradiation. This is one of the disadvantages of thin-layer chromatography.
In order to understand the principles of thin-layer chromatography, the properties of silica gel as an adsorbent must be studied. In addition, the understanding of the mobile phase and Rf values will help in synthetic studies. The theory is not difficult, so you should understand the characteristics and properties of TLC, including its advantages and disadvantages.