Chromatography is a method of separating substances completely. There are several types of chromatography, of which column chromatography is the most frequently used in laboratories that deal with organic compounds.
A type of adsorption chromatography is column chromatography. It uses an adsorbent called silica gel to separate several organic compounds that are mixed together.
Column chromatography is an experimental operation that is performed every day in laboratories that handle organic compounds, such as those that require organic synthesis and structure determination of compounds.
So what are the principles of separating organic compounds by column chromatography? What are the methods and procedures of column chromatography? We will explain these in this article.
Table of Contents
- 1 Normal-Phase Chromatography for Complete Separation of Organic Compounds
- 2 The Speed of Movement Through the Stationary Phase (Silica Gel) Depends on the Substance
- 3 Highly Polar Compounds Interact with the Stationary Phase, the Adsorbent
- 4 Fill with Silica Gel and Pour Developing Solvent
- 5 Understand the Principles and Learn How and What to Do with the Experiment
Normal-Phase Chromatography for Complete Separation of Organic Compounds
Adsorption chromatography is a technique for separating chemicals by using their ability to adsorb substances. There are several types of adsorption chromatography, one of which is column chromatography. Column chromatography is also known as normal-phase chromatography.
Strictly speaking, there are differences, but in general, adsorption chromatography and normal-phase chromatography can be considered the same thing. Column chromatography is an instrument that looks like the following.
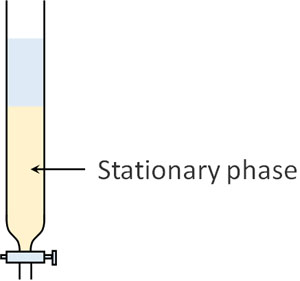
In column chromatography, a substance is placed in a column (a small tube) as a stationary phase. The compounds gradually move forward as they are adsorbed by the stationary phase.
The Speed of Movement Through the Stationary Phase (Silica Gel) Depends on the Substance
Silica gel is used as the stationary phase in column chromatography. Sometimes alumina is used in column chromatography, but silica gel, not alumina, is the most common choice. There is no need to consider the use of alumina.
Silica gel is solid and is used as a stationary phase in column chromatography. The structural formula of silica gel is as follows.
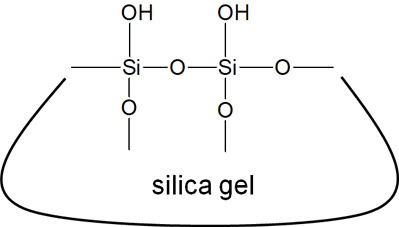
As you can see from this structural formula, silica gel is extremely polar. There are a lot of oxygen atoms with high electronegativity around the silicon.
Silica gel has these properties. When a compound passes through a column filled with silica gel, there is a difference in its speed of movement. This difference in velocity is used to separate the compounds.
HPLC (High Performance Liquid Chromatography) Is Reverse-Phase Chromatography
Note that among the also well-known chromatographs, HPLC (high-performance liquid chromatography) and column chromatography are different.
Column chromatography utilizes highly polar substances such as silica gel and alumina as the stationary phase. Thus, column chromatography, which uses a highly polar substance as the stationary phase, is normal-phase chromatography.
On the other hand, reversed-phase chromatography uses less polar substances (highly hydrophobic substances) as the stationary phase. The nature of the substances used for the stationary phase (column) is different.
- Normal-phase chromatography: uses a highly polar substance as the stationary phase.
- Reversed-phase chromatography: uses a highly hydrophobic substance as the stationary phase.
In HPLC (high performance liquid chromatography), reversed-phase chromatography is the main method. Think of it as different in nature from column chromatography.
Highly Polar Compounds Interact with the Stationary Phase, the Adsorbent
When using column chromatography, why do different compounds move at different speeds through the silica gel? This is because they interact with the silica gel. In other words, the compounds are adsorbed by the silica gel.
Water and oil do not mix. This is because water has a high polarity and oil has high fat solubility. Compounds with high polarity are soluble in water. On the other hand, even compounds that are highly fat-soluble and insoluble in water are soluble in oil. Substances with the same properties have a high affinity for each other.
In this way, substances with high polarity tend to interact with silica gel, which is also highly polar. In other words, they will be adsorbed by the silica gel. As the solvent flows, the compound is adsorbed by the silica gel and gradually moves forward, while leaching into the organic solvent of the mobile phase.

In contrast, what about less polar compounds (highly hydrophobic compounds)? They do not interact with the highly polar silica gel and will not be adsorbed by it. As a result, they move forward quickly when the solvent flows.
This is the reason why the speed of progress of organic compounds differs when organic solvents are poured into a cylinder filled with silica gel.
The Speed (Degree of Leaching) Differs Depending on the Polarity of the Organic Solvent in the Mobile Phase
Also, in column chromatography, you have to decide on a mobile phase. The stationary phase is a choice of silica gel. However, you have to decide which type of solvent to flow.
The most common organic solvents used in column chromatography are ethyl acetate and hexane.
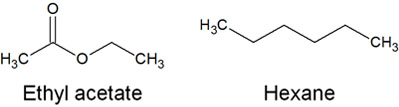
As you can see from the structural formula, ethyl acetate has a high polarity. On the other hand, hexane has a lower polarity. In column chromatography, ethyl acetate and hexane are generally mixed together. The mobile phase (organic solvent) used in column chromatography is called the developing solvent.
Due to the high polarity of ethyl acetate, if the ratio of ethyl acetate is increased in the developing solvent, the substance will quickly pass through the silica gel. Even though the compound is adsorbed on the silica gel, it is still easily dissolved in the highly polar ethyl acetate and moves quickly through the column chromatography.
On the other hand, what if the ratio of hexane is increased? Because hexane is highly hydrophobic, it is difficult for a compound to dissolve in an organic solvent with a high hexane ratio. As a result, the higher the percentage of hexane, the slower the compound will move through the column.
When creating a developing solvent, the ratio of ethyl acetate and hexane must be determined based on an understanding of their properties and characteristics.
Determine the Mobile Phase (Developing Solvent) from the Rf-Value of Thin-Layer Chromatography
How do we decide on a developing solvent? Before performing column chromatography, there is one experiment that must be done. That is thin-layer chromatography (TLC). Column chromatography without thin-layer chromatography is not a common practice in chemistry.
TLC is a thin glass plate with silica gel on it. When you do thin-layer chromatography, you spot the solution you want to measure. You then create a developing solvent and immerse the TLC in the developing solvent so that the compounds can be separated.
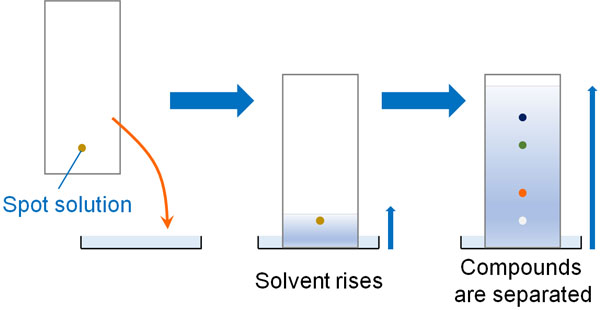
The Rf-value can be measured for each compound. The speed of a compound in silica gel is called Rf-value. The Rf-value is shown below.
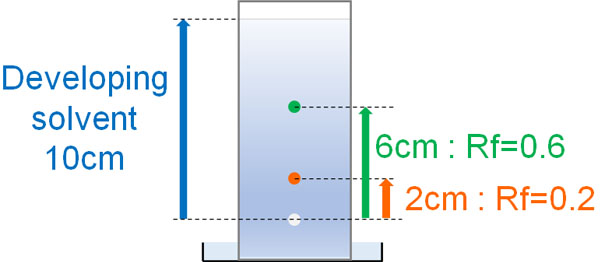
The Rf-value depends on the developing solvent. We adjust the ratio of ethyl acetate to hexane so that the target compound has an Rf-value of about 0.3.
The higher the Rf-value, the faster the compound moves through the silica gel, and there is a risk of separation of the compound along with other impurities in the solution. On the other hand, if the Rf-value is too low, the target compound will not come out of the column even though the developing solvent is flowing for a long time.
So let’s do a TLC and find a developing solvent that gives an Rf-value of about 0.3. Then, pour the solvent into the column.
-The Developing Solvent Can Be Dichloromethane and Methanol
The most common method of column chromatography is to use an organic solvent mixed with ethyl acetate and hexane. In some cases, however, other developing solvents may be used.
In addition to ethyl acetate and hexane, a mixture of dichloromethane and methanol is also used as a developing solvent. Sometimes the dichloromethane/methanol combination is better for separating compounds with very high polarity.
Fill with Silica Gel and Pour Developing Solvent
We have explained the principles and characteristics of column chromatography. So, when you actually do column chromatography, how does it work?
First, you have to put a silica gel in the column. Then, the compound is placed on top of the silica gel. Once you have completed the preparations up to this point, you just need to flow the developing solvent. As the mobile phase flows, the compound will gradually move forward as well.
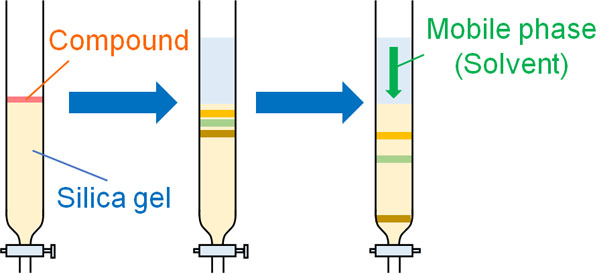
Naturally, the mobile phase, the organic solvent, comes out of the end of the column. The organic solvent is collected in test tubes. Once the solution containing the target compound is collected, the separation of the compound is complete.
Now let’s consider the compounds estriol, estradiol and estrone as an example.
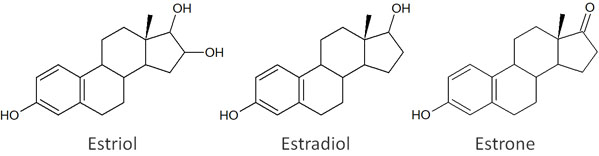
Suppose you have a solution that contains a mixture of these compounds. What will be the difference in speed when separating these compounds by column chromatography?
The polarity is estriol > estradiol > estrone, in that order. If we focus on the hydroxy group (-OH), the nature of the substance is easy to predict.
In column chromatography, the higher the fat-soluble compound, the less affected it is by silica gel. In other words, we can expect the substances to flow out in the following order.
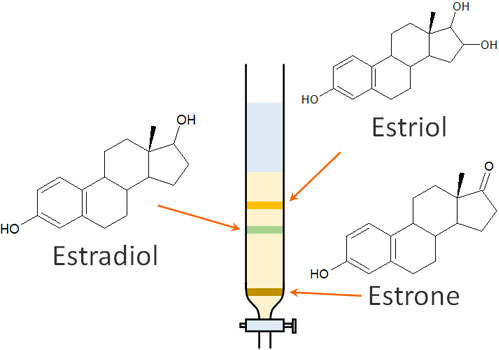
Column chromatography is an experimental operation that focuses on the polarity of a substance and separates organic compounds. Once you understand the principle, you will be able to predict the speed at which the compounds will flow.
If the Compound Is Not Separated, Change the Length of the Column
However, the compounds may not be separated in actual chemical experiments. The ability to separate compounds is an advantage, but in some cases, the disadvantage of column chromatography is that you have to consider the experimental method.
A common example of this is the close Rf-values between compounds, making it difficult to separate them, as shown below.

In this case, the most obvious thing to do is to increase the amount of silica gel you put into the column. The longer the stationary phase (silica gel), the easier the compounds will separate.
For example, in a 100m run, a difference of one second is a big difference. On the other hand, if you run a full marathon of 42km, it is common to see a time difference of 10 minutes or more. The longer the distance, the bigger the difference will be.
Lowering the Rf-Value Will Prevent Failure
Alternatively, you can use a lower Rf-value. Even if it’s the same column length, the lower the Rf-value, the easier it is to separate the compounds.
For example, if you’re running 100 meters and there are no obstacles, the difference in time is minimal. On the other hand, what if there is a pond, a large block or a maze within 100m? The total time will be completely different for each person.
Similar to this principle, the lower the Rf-value, the higher the separation capability of the compound. Of course, there is a disadvantage that the movement speed of the compounds becomes very slow, so the compounds do not show up easily even when the developing solvent is flowing. However, it will allow you to separate compounds more reliably.
If you can’t separate compounds by column chromatography, you should apply both a longer stationary phase and a lower Rf-value. Otherwise, you will fail to separate the compounds.
Understand the Principles and Learn How and What to Do with the Experiment
Column chromatography is a widely used experimental technique in laboratories that handle organic compounds because of its ability to separate compounds. Therefore, it is important to understand the principle, characteristics, advantages and disadvantages before conducting experiments.
Use silica gel as a stationary phase, and understand that silica gel has a high polarity. Then the higher the polarity of the compound, the more it will be adsorbed on the silica gel, and the slower the rate will be.
You also have to determine the mobile phase. We use organic solvents to create the developing solvent. First, the ratio of ethyl acetate and hexane is determined. The higher the ratio of ethyl acetate, the more easily the compound dissolves in the solvent, and the faster the rate. In other words, the Rf-value will be higher.
After learning these things, we decide on a developing solvent, measure the Rf-value, and perform column chromatography. Once you understand these experimental procedures, you will be able to separate only the target compound even when many compounds are mixed together.