Molecules may attract or repel each other. This is called an interaction. In chemistry, an interaction refers to molecules that affect each other. Also, in many cases, interactions refer to a state in which molecules are attracted to each other.
Interactions are different from molecules bonding together to form covalent bonds. If a new bond is created by an organic chemical reaction, a new molecule is created. This bond is a covalent bond. Instead, interaction is that molecules try to attract each other and stick together, while maintaining their original structure.
These are called intermolecular forces (IMF: intermolecular interaction). Molecules interact with each other and try to stick together.
There are several types of intermolecular forces. Typical examples are dipole interactions, hydrogen bonds, van der Waals forces, and hydrophobic interactions (or hydrophobic effects). There are many different types of intermolecular interactions, so we will explain how each type of bond works.
Table of Contents
- 1 Polarization Caused by Differences in Electronegativity
- 2 Hydrogen Bonds Occur with Oxygen, Nitrogen and Fluorine
- 3 Van der Waals Forces that Bind Molecules Together
- 4 Increase in Entropy Due to Hydrophobic Interactions (Hydrophobic Effect)
- 5 Molecules Are Attracted to Each Other Due to Intermolecular Forces
Polarization Caused by Differences in Electronegativity
Broadly speaking, non-covalent bonds are intermolecular forces. Although the molecules are independent of each other, they are still attached to each other by other forces, without forming a strong covalent bond. Therefore, when energy is applied from outside, the attached molecules detach.
When learning about intermolecular interactions, the first step is to understand polarization. Since we learn polarization in high school chemistry, we can understand it without any problem. Polarization is what creates differences in electrical density due to differences in electronegativity.
For example, oxygen, nitrogen, and halogen atoms are known as atoms with strong electronegativity. When these atoms are bound to carbon, the same molecule can be divided into positive and negative charges.
Examples of polarization are known to include water, ammonia, and hydrogen chloride (HCl).
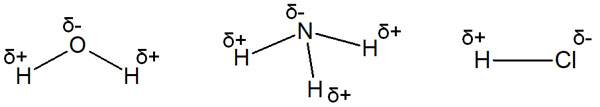
Polarization is the phenomenon of the same molecule being split into positive and negative charges. All you need to understand is that the difference in electronegativity results in a positive and negative charge.
Dipole Moments in Polarization: Polar and Nonpolar Molecules
Even though polarization produces a difference in charge, different molecules produce different degrees of charge polarization. The strength of the polarity (the degree of charge polarization) is called the dipole moment.
The greater the polarization due to differences in electronegativity, the stronger the polarity. As a result, the dipole moment becomes larger.
In a dipole moment, we write a vector from negative to positive charge. There is no need to think too much about the meaning of the arrows. The point is to understand that the dipole moment describes how large or small the polarization is.
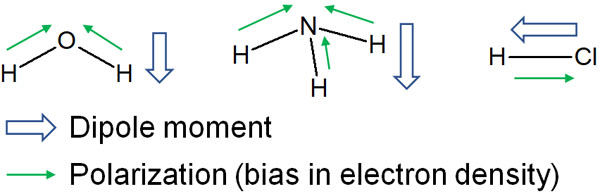
For reference, in organic chemistry, it is important to understand the movement of electrons. Starting from an atom with a negative charge, it attacks other molecules and causes an organic chemical reaction.
For this reason, the dipole moment is not used in organic chemistry. When we describe polarization (bias in electron density), we write an arrow from positive to negative. In a dipole moment, the direction of the arrow is opposite. It is common in organic chemistry to think of negatively charged atoms as the starting point.
-The Concept of Polar Molecules (Permanent Dipoles) and Nonpolar Molecules
So, does an atom with high electronegativity always have polarity when it is bonded? This does not necessarily mean that they are polarized. There are some molecules that are not polarized. Molecules that are symmetrical do not have polarization.
For example, carbon dioxide (CO2) is symmetrical, so it is not polarized. Similarly, methane, which has a tetrahedral shape, is not polarized.
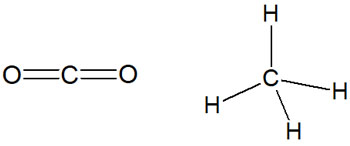
Polarized molecules, such as water and ammonia, are called polarized molecules (permanent dipoles). On the other hand, non-polar molecules such as carbon dioxide and methane are called nonpolar molecules.
Molecules Attract Each Other Through Dipole Interactions: Keesom Interactions
What happens when molecules become polar due to polarization? Molecules are attracted to each other. Specifically, the positive and negative charges interact with each other. On the other hand, positive and positive charges repel each other. The same is true for negative and negative charges.
In such cases, the attraction of these charges to each other due to polarization is called dipole interaction.
When the dipoles are attracted to each other by the charge of the ions, this is called ion-dipole interaction. When dissolved in a solution, compounds that exist as ions will attract dipoles.
On the other hand, when dipoles are attracted to dipoles, it’s called dipole-dipole interaction (or Keesom interaction). Compared to ions, the difference in charge due to the dipoles is small. Therefore, the force of the dipole-dipole interaction is weaker than that of the interaction between ions and dipoles.
Interaction by Induced Dipoles: Dipole-Induced Dipole Interaction
So neutral molecules do not interact? If there is no dipole, there is no positive or negative charge, so you might think that neutral molecules do not interact. However, molecules with dipole and neutral molecules do interact and attract each other.
What happens when a polar molecule is placed near a neutral molecule? Due to the effect of the dipole, the neutral molecule will have an intramolecular charge bias. In other words, a dipole is created.
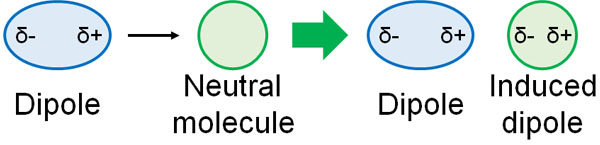
When a dipole is produced under the influence of another dipole (a polar molecule), it is called an induced dipole. When an induced dipole is produced, it interacts with the dipole and pulls on each other. This is known as dipole-induced dipole interaction (or Debye interaction).
In polarized molecules, even neutral molecules will attract each other by inducing the induced dipole.
London Dispersion Force Induced Dipole
In contrast, do neutral molecules (non-polar molecules) ever interact with each other? Actually, even non-polar molecules will interact and attract each other.
It may seem odd that non-polar molecules can interact with each other. However, even in the same molecule, the electrons do not stay in one place, they exist in various places in the molecule.
So, let’s try to capture a single moment in time. Just like taking a picture of a moment in time, if you capture a specific moment in time, you can discover the bias of electrons in a molecule. As a result of this instantaneous electron bias, even neutral molecules become momentarily polarized and have a dipole.
These instantaneous dipoles affect other nonpolar molecules, causing induced dipoles. As a result, even if only neutral molecules exist in space, they will interact with each other. This is called the London dispersion forces.
Neutral substances are known to attract each other, which is called the van der Waals force. It is important to understand that the van der Waals forces of non-polar molecules are mostly due to the London dispersion forces.
Hydrogen Bonds Occur with Oxygen, Nitrogen and Fluorine
Once you learn about these polarization and dipole moments, you will be able to understand hydrogen bonds. A hydrogen bond is literally a bond that attracts and bonds with each other by interacting with hydrogen. While not as strong as a covalent bond, a hydrogen bond produces a strong force.
The greater the degree of polarization and the greater the dipole moment, the stronger the force of attraction. When considered in terms of the degree of polarity, the polarization tends to increase when hydrogen is bonded to an atom with a high degree of electronegativity.
Specifically, when hydrogen atoms are bonded to oxygen (O), nitrogen (N), and fluorine (F) atoms, the polarization is very large. As a result, the dipoles created in the molecule strongly attract each other, and a strong bond is formed through the dipole-dipole interaction.
Hydrogen bonding is a type of dipole-dipole interaction. However, among the dipole-dipole interactions, hydrogen bonds are highly polarized and strongly attracted to each other. A hydrogen atom is attracted to another oxygen, nitrogen, or fluorine atom, resulting in the formation of a hydrogen bond.
A hydrogen bond can attract another molecule, or it can form a hydrogen bond inside a molecule. It is as follows.
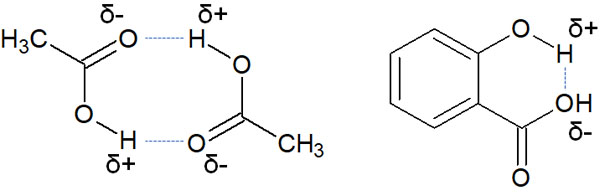
Although it is a bond between dipoles, hydrogen bonding is a strongly attractive interaction between hydrogen atoms and atoms with high electronegativity.
Proteins and DNA Are Hydrogen-Bonded
Why do we consider only hydrogen bonds separately among dipole interactions? This is because the intermolecular interactions (intermolecular forces) caused by hydrogen bonds are very important.
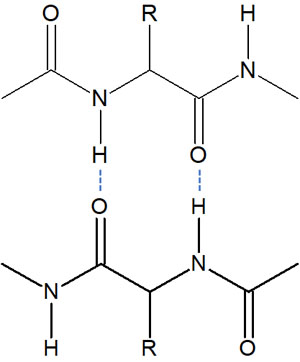
For example, there are many proteins in our bodies. Why do proteins have a particular shape? This is because of the hydrogen bonds involved. Proteins are formed when many amino acids are combined together. A protein can be described as a single long chain of amino acids.
However, if they are the same protein (same sequence of amino acids), the proteins will all have the same structure by folding. The reason for this is that hydrogen bonds interact and attract each other inside the protein.
The same can be said for DNA, which is known to be formed by two base pairs. These are connected by hydrogen bonds, resulting in the double-helix structure of DNA.
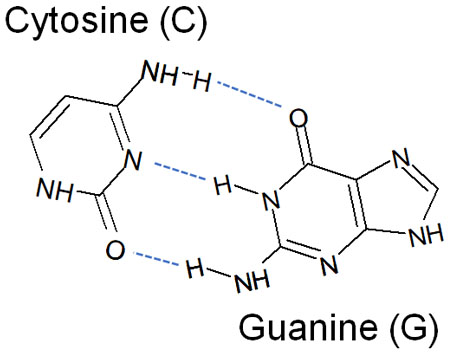
Understanding hydrogen bonding is essential, not only for bonding in molecules and organic chemical reactions but also for understanding what happens in living organisms.
Van der Waals Forces that Bind Molecules Together
Compared to the dipole interactions and hydrogen bonds we have described, the van der Waals force is weaker in its attraction to each other.
We have explained that the occurrence of polarization leads to dipole interactions and hydrogen bonds. However, even neutral molecules have the ability to attract each other. This is the van der Waals force as mentioned above. As already explained, most of the forces that attract nonpolar molecules to each other are due to the London dispersion forces.
London dispersion forces produce induced dipoles, as a result of which neutral molecules are attracted to each other as van der Waals forces.
Van der Waals forces exist in all substances. This means that all molecules, even nonpolar ones, will attract each other.
Repulsion by Leonard-Jones Potential
An important factor in van der Waals forces is the distance between molecules. Neutral molecules attract each other, but the farther they are from each other, the weaker the attraction. It is easier to attract things that are close to each other than attract things that are far away.
So, the closer the molecules are to each other, the stronger the force of the Van der Waals force becomes? Actually, the closer they are, the more repulsive force is applied.
Molecules have electrons. The electrons repel each other, so when they get too close together, the electrons repel, and the energy becomes higher.
If the neutral molecules are far away from each other, there is no attraction, and the energy state is high (the energy is close to zero). On the other hand, if the molecules are too close to each other, the energy is high because of the repulsion that is created. The van der Waals force favors a state that is neither too far nor too close.
If the distance between neutral molecules is defined as r, then the attraction of the Van der Waals force is inversely proportional to the 6th power of r. The closer the distance is, the stronger the attraction is.
On the other hand, the repulsive force of the van der Waals force is inversely proportional to the 12th power of r. The closer the distance between the molecules, the stronger the repulsive force becomes. Try to understand this law as such.
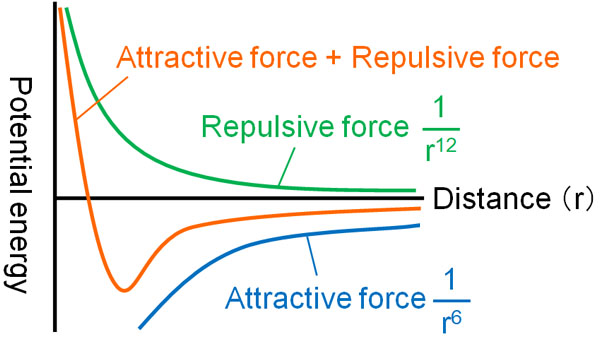
The Leonard-Jones potential is the one that takes into account both the attraction and the repulsive of the Van der Waals force. The figure above shows Leonard-Jones potential.
The curve is steeper to the 12th power of r than to the 6th power of r. Therefore, when the molecules are at a good distance from each other, they attract each other, resulting in lower energy. Since the attraction is stronger than the repulsive force, they will attract each other by van der Waals forces.
On the other hand, if the distance is too close, the energy due to repulsive force will be very high. Since the repulsive force is stronger than the attractive force, when the distance is too close, they repel each other.
The concept of Leonard-Jones potential is difficult to understand. But when you realize that the Leonard-Jones potential is a summary of the attractive and repulsive forces between molecules, you will be able to understand what it means.
Increase in Entropy Due to Hydrophobic Interactions (Hydrophobic Effect)
In terms of intermolecular interactions (intermolecular forces), hydrophobic interactions are also important as a force of attraction between molecules. Let’s understand that hydrophobic interactions (hydrophobic effect) are the forces of attraction between fat-soluble molecules to each other.
The most familiar liquid in our lives is water. Water and oil do not mix with each other because they are completely different in nature. If there are two oils, they will stick to each other to avoid the water.

The hydrophobic effect is the force that causes oils to try to stick to each other. These hydrophobic interactions (hydrophobic effect) are also related to entropy increase.
If left unchecked, the progression to a messy state is called entropy increase. If you neglect your garden, weeds will grow, and it will become rough. If you don’t keep things tidy, your room will become cluttered. According to the laws of nature, everything moves to become cluttered.
This is the same with oil. When the oil is in water, water will be present around the oil. But what if the oils stick together? If we focus on the water that was around the oil (hydrophobic molecules), the number of water molecules that can move freely will increase.
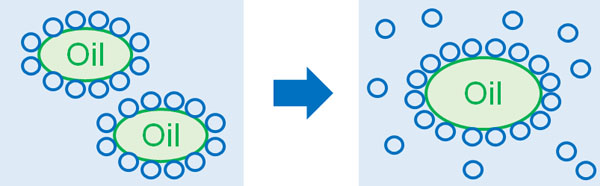
Rather than having several hydrophobic molecules floating around in the water, the number of water molecules that can move around freely is greater when these oils exist as a single unit. The more water molecules can move around, the more water entropy (clutter) is increased. This is one of the reasons why hydrophobic interactions occur.
Micelle Formation Is an Application of Hydrophobic Interactions
When you study chemistry, you will study micelles. Micelles are compounds that have hydrophilic and hydrophobic parts in the same molecule. It is a so-called surfactant. As a familiar example, detergent is a surfactant. Detergents make micelles.
When a surfactant is placed in water, the hydrophilic part of the surfactant sticks to the water. It interacts positively with water through hydrogen bonding.
On the other hand, the hydrophobic group tries to avoid contact with water as much as possible. This is the so-called hydrophobic interaction, which reduces the contact area with water. The hydrophobic parts also attract each other by van der Waals force.
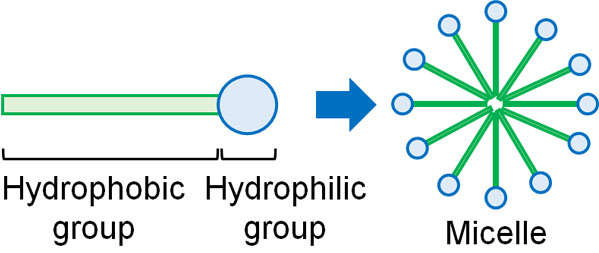
As a result, the micelles begin to form aggregates in the water. Thus, the micelles are stabilized in water.
When micelles are formed in water by the surfactant, the oil (sebum and other dirt) is taken up by the hydrophobic part of the micelles. As a result, the micelles can be used as a detergent to remove grease and dirt. The most obvious application of micelles is in the field of detergents, but micelles are also used in many other fields such as medicine and food.
Molecules Are Attracted to Each Other Due to Intermolecular Forces
There are several types of bonds between molecules, including covalent bonds and ionic bonds (Coulomb forces).
Among these bonds, intermolecular interaction (intermolecular force) is the force of attraction between molecules that causes them to stick to each other. Although not as strong as a covalent bond, the molecules are attracted to each other. In these intermolecular interactions, the following are particularly important.
- Dipole interactions
- Hydrogen bond
- Van der Waals force
- Hydrophobic interaction (Hydrophobic effect)
Once you understand molecular polarization, you can understand why dipole interactions, hydrogen bonds, and van der Waals forces occur. Although hydrophobic interactions are a slightly different concept, it is essential when learning about molecular interactions.
Learn about these types and the differences between them, and make sure you understand the effects of intermolecular forces.