Chromatography is very important in the separation of compounds. Adsorption chromatography is one of the most common methods used.
Adsorption chromatography is also called normal phase chromatography. Although they are not exactly the same, for the sake of clarity, we can understand that adsorption chromatography is almost the same as normal phase chromatography.
Compared to methods such as HPLC (reversed-phase chromatography), normal phase chromatography is less common. However, some people, such as those who do synthetic research in organic chemistry, use normal phase chromatography on a daily basis. For some people, it is a very important method.
Although adsorption chromatography is an excellent method for separating compounds, it is difficult to understand the principles and characteristics of its use without understanding its application. In this section, we will discuss the concept of normal phase chromatography.
Table of Contents
Separate Multiple Substances by Normal-Phase Chromatography
When you’re doing research, you don’t want to have multiple compounds mixed together. Even if you try to determine the structure, it is not possible to distinguish which is the data of the target compound because it is not a single compound.
Therefore, it is necessary to separate substances in advance. While there are several ways to separate compounds, one very accurate method is chromatography. Adsorption chromatography (normal phase chromatography) is a method of separating substances.
The compounds are dissolved in an organic solvent and by doing normal phase chromatography, they are made into a single substance.
So how do we separate the substances? As an image, let’s think of a marathon. For example, if the following two people were to run a marathon at the same time, which one would be faster?
Perhaps fat women seem to have slower feet. The same is true for compounds, and when flowing with a liquid (organic solvent), the speed at which it flows is different for each compound. This difference is used to separate substances.
Silica Gel Is Generally Used As a Stationary Phase
How do the compounds show differences in speed? In chromatography, we always prepare a stationary phase. By passing the compound through the stationary phase, the compound produces a difference in speed. The stationary phase is placed in a thin tube, and the compound is passed through it.
Depending on what stationary phase is being prepared, the name of the chromatography changes. For adsorption chromatography, the most common choice is silica gel.
In our daily lives, we use silica gel granules as desiccants. On the other hand, the silica gel used in chemical experiments is very fine and silky. After filling the silica gel into a tube, we pour a solvent with the dissolved compound.
Although there are several stationary phases such as silica gel, alumina, and activated carbon, we use silica gel in normal phase chromatography unless there is a good reason to use it.
Highly Polar Compounds Are Slowed Down by Interactions
So why does flowing the solvent after filling the silica gel make a difference in the speed of compounds? This can be understood by focusing on the structure of the silica gel.
The silica gel has a very large number of hydroxyl groups. In other words, there are a lot of -OHs on the surface of the silica gel. The oxygen atoms have a high degree of electro-negativity and are highly polar. It is important to understand that silica gel is a highly polar substance because it possesses a lot of hydroxyl groups.
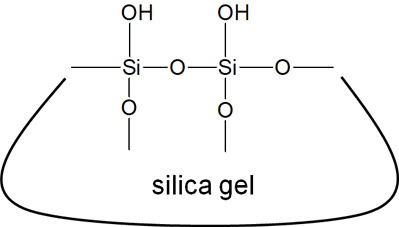
The closer the properties of a compound are, the more likely it is to stick together. For example, water and oil do not mix. This is because water has a strong polarity and oil has a low polarity.
The same is true for compounds, which have a strong or weak polarity. Since silica gel is highly polar, the more polar a compound is, the more easily it is adsorbed onto silica gel. On the other hand, compounds with low polarity (high hydrophobicity) are less likely to be adsorbed by silica gel.
As a result, when the silica gel is filled and the solvent flows, the compounds can be separated.
The compounds will flow with the solvent. But what if there is a highly polar silica gel? The highly polar compounds are adsorbed by the silica gel, and they move forward little by little. Even if it tries to move forward, the compound cannot move easily because of the silica gel adsorbent.
Just as a human being walks through a muddy swamp at a slow speed, the speed of movement slows down when there are adsorbed substances.
On the other hand, what about compounds that are highly hydrophobic? In this case, there is less interaction with silica gel. In other words, the adsorption effect by the silica gel is weak. As a result, it can move forward quickly.

Naturally, different compounds have different degrees of polarity. As a result, each compound moves at a different speed, and as a result, even if a number of compounds are mixed together, they can be separated.
The Mobile Phase Will Be the Solvent for the Organic Compounds
In addition, the mobile phase must be determined in adsorption chromatography. The mobile phase in normal phase chromatography is an organic solvent. For example, the following organic solvents are used.
- Ethyl acetate
- Hexane
- Methanol
- Dichloromethane
- Ethanol
- Acetone
Although there are many other types of organic solvents, the most common solvents used in normal phase chromatography are ethyl acetate and hexane. Among the organic solvents, ethyl acetate is highly polar, as shown by its structural formula. On the other hand, hexane has a low polarity.
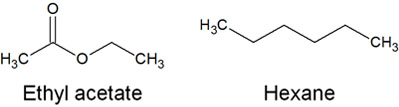
No matter how polar the organic compounds are, if you use a solvent with high polarity, adsorption on silica gel is unlikely to occur. Even if you think the compound is adsorbed on silica gel, it will soon dissolve into ethyl acetate and move forward with the solvent.
Therefore, ethyl acetate and hexane are mixed together. The ratio of hexane can be freely adjusted to 30%, 50%, or 80% of the mixture.
What happens if the ratio of hexane is high? Organic compounds with high polarity cannot dissolve into the mobile phase (liquid), and their speed of progress becomes slower.
Most compounds are not soluble in hexane because of its high fat solubility. On the other hand, most compounds are soluble in ethyl acetate. Therefore, the ratio of ethyl acetate to hexane is adjusted to ensure that organic compounds pass through the silica gel tube at a moderate speed.
Although the optimum ratio of ethyl acetate to hexane varies with the compound, the organic solvent of the mobile phase is determined in this way.
There Are Different Types of Adsorption Chromatography
So what are the different types of adsorption chromatography? Reversed-phase chromatography is very commonly used in HPLC, which is very often used in chromatography. Reversed-phase chromatography utilizes a highly hydrophobic stationary phase.
However, we will not consider here reversed-phase chromatography, but rather normal phase chromatography using a stationary phase with high polarities, such as silica gel. The following types of normal phase chromatography are known to be used in chemical research.
- Thin-layer chromatography (TLC)
- column chromatography
Since the principle of both is adsorption chromatography, the concept is the same. However, the purpose of the use is different.
Thin-Layer Chromatography (TLC) to Distinguish Between a Number of Substances
In chemical experiments, you have to find out if it is a single compound or a mixture of substances. For example, it is quite common for organic compounds to be synthesized and produce byproducts. And even when you extract a compound, it’s common to find several compounds mixed together.
The first step is thin-layer chromatography (TLC). As the term thin layer implies, it utilizes a thin plate. It is a glass plate with silica gel attached to it, and the following are actual TLC plates.

First, spot a solution of the compound on the TLC. Then it is immersed in the mobile phase (a liquid mixture of ethyl acetate and hexane). Gradually, the liquid rises from the bottom to the top. Along with that, the compound also climbs to the top.
However, as mentioned earlier, different compounds have different polarities and therefore rise at different speeds. As a result, thin layer chromatography will cause the compounds to separate on the TLC.
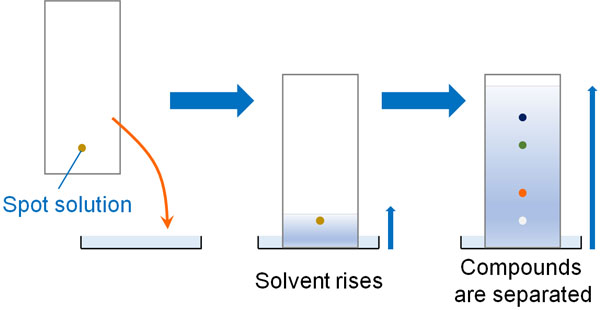
Thin-layer chromatography allows us to determine how many compounds are in the solution. For example, if there are three spots of organic compounds by thin-layer chromatography, we can understand that there are three compounds mixed in the solution.
Even if you do TLC, you will not be able to separate the compounds. Thin-layer chromatography is a technique to determine how many compounds are in the mixture and whether the mobile phase is optimal.
Separation of Materials by Column Chromatography
In this way, we check how much of the compound is present in the solution. The mobile phase (ethyl acetate to hexane ratio) is also checked to ensure that it is optimal.
The compounds are then separated. Column chromatography is used for the separation of compounds. The narrow tube is the column, and adsorption chromatography is performed on the column, hence the name column chromatography. Especially in synthetic organic laboratories, we do column chromatography every day.
First, the column is filled with silica gel. Next, the compound is placed on top of the silica gel. After that, the solvent (mobile phase) is poured down.
As with thin-layer chromatography, the speed at which the column flows depends on the compound. Using this property, the compounds are collected in sequence as they emerge from the column.
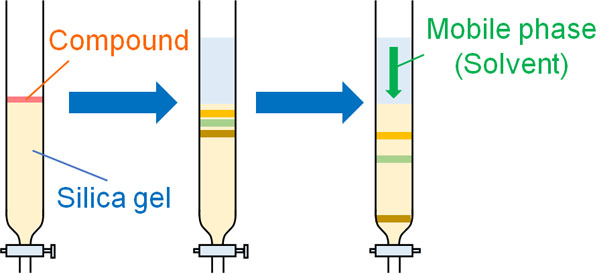
The liquid is collected in test tubes and evaporated. In this way, even if there are several compounds mixed together, they will become a single compound by normal phase chromatography.
For example, let’s say you have a mixture of three compounds: estriol, estradiol and estrone.
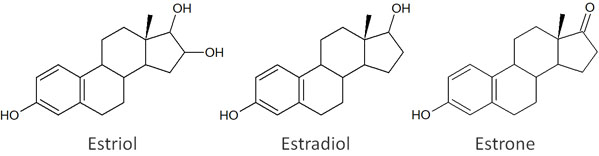
For these compounds, the order of polarity strength is estriol > estradiol > estrone. The strength of polarity can be determined by the presence of hydroxy groups (-OH). Also, the more hydroxy groups there are, the stronger the polarity will be.
The more polar a compound is, the slower it travels through the column as it interacts with the silica gel. Therefore, in column chromatography, we have the following.
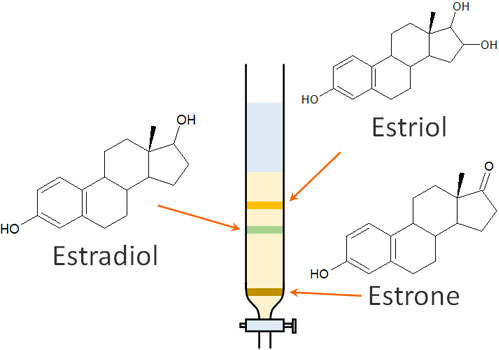
It doesn’t matter if it is a mixture of multiple substances. Column chromatography can be performed to separate them into a single compound. This is the nature of column chromatography.
Understand the Nature and Application of Adsorption Chromatography
Here we have explained the principles and characteristics of adsorption chromatography in as clear a manner as possible. Although there are several types of adsorption chromatography, it is generally referred to as normal phase chromatography. Strictly speaking, they are different, but for the sake of clarity, let’s understand them that way.
You also need to learn about the stationary and mobile phases in normal phase chromatography. For the stationary phase, silica gel is mainly used. Silica gel is highly polar, so the more polar a substance is, the more it interacts with it, the harder it is to move forward.
Adsorption chromatography is used to separate compounds using these properties. The two main types of adsorption chromatography are thin-layer chromatography (TLC) and column chromatography. Because they have different roles, the two must be used in different ways.
In laboratories that work with organic compounds, adsorption chromatography is used daily in experiments. Therefore, it is especially essential for these laboratories to understand the principles of adsorption chromatography.