In organic chemistry, we often use cyclic compounds. A typical cyclic compound is cyclohexane, and these compounds are used in synthetic reactions.
Cyclic compounds have strain. They are in a high energy state due to steric hindrance and other factors. The more strain there is, the more unstable the state is, and the more reactive the compound becomes. Understanding the strain will also help us to understand the conformation of the compound.
Cyclohexane is particularly important in conformation. Organic chemistry needs to be considered in three dimensions, not in a plane. There are many things in cyclohexane that must be considered in stereochemistry.
Therefore, we will explain the strains that are important in cyclic compounds, and then we will check the stability and conformation.
Table of Contents
There Are Strains and Steric Hindrances in Cyclic Compounds
In compounds such as alkanes, the axis of the molecule can rotate to create a variety of shapes. However, in cyclic compounds, the axis does not move freely as in alkyl chains. The carbon chain is fixed.
The compound has an ideal form. However, if the movement is restricted, as in the case of a cyclic compound, it will cause strain in the molecule. As a result, the energy possessed by the molecule will be higher.
The following types of strains are known.
- Angle strain (bond angle strain)
- Torsional strain
- Steric strain
The carbon atoms in cycloalkanes are sp3 hybrid orbitals. In this case, a bond angle of 109.5° is an ideal angle and a stable structure. For example, methane has a bond angle of 109.5°.
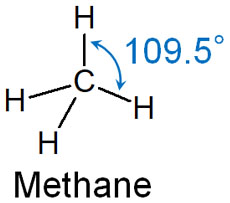
However, in cyclic compounds, the bond angle may not be 109.5°. In this case, bond angle strain occurs.
The torsional strain is also known in stereochemistry. When molecules bond with each other, the ideal dihedral angle (θ) is 60°.
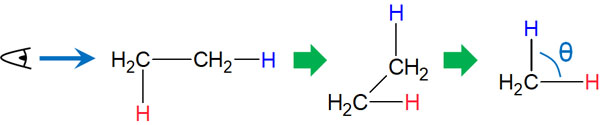
When the dihedral angles overlap, the electrons involved in the bonding are repelled. It also causes steric hindrance. As a result, the energy becomes higher.
In addition, when larger substituents are in close proximity, the steric hindrance increases. In cycloalkanes, the ring-shaped nature of the molecule frequently induces steric hindrances.
Cyclopropane Is Highly Strained
What kind of strain is produced in cyclic compounds? Cyclopropane, for example, is known as a cyclic compound. A three-carbon compound is a propane, and in cyclopropane, it is a cyclic compound.
Since cyclopropane is triangular, the bond angle is 60°. This is a significant deviation from the ideal bonding angle of 109.5°. As a result, cyclopropane has a large angle strain.
In addition, if you write cyclopropane in the Newman projection, you can see that the C-H bonds overlap. The dihedral angle is not 60°, but 0° in cyclopropane. As a result, cyclopropane has a torsional strain.
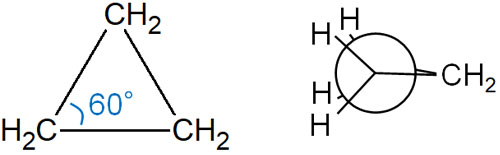
Because of these strains, cyclopropane is known to have very high energy. Cyclopropane is an unstable compound and is easy to react chemically.
Cyclobutane and Cyclopentane Cause Less Strain
On the other hand, what about cyclobutane and cyclopentane? In these compounds, the bond angle is larger than in cyclopropane and is closer to 109.5°.
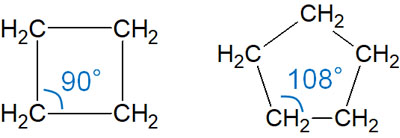
However, the bond angle is still not 109.5°. Therefore, these compounds have an angle strain. However, they are lower in energy and more stable than cyclopropane.
These cyclic compounds also have torsional strain. Since the bond and dihedral angles are fixed, cyclic compounds have higher energy than alkyl chains.
Stability and Chair Conformation of Cyclohexane
On the other hand, what about cyclohexane? Cyclohexane has an angle of 120°, and you would think that it would have angle strains. However, cyclohexane has a form of chair conformation. Although the bond angle is 120° in the plane, cyclohexane has a bond angle of 109.5° when considered in terms of stereochemistry.
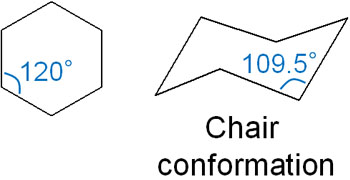
Also, in the cyclohexane chair conformation, the dihedral angle is 60°. As a result, there is no torsional strain. The following is a Newman projection of cyclohexane.
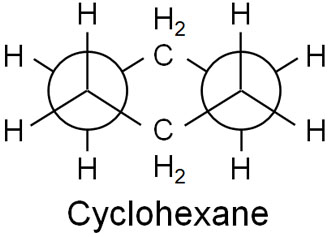
In cyclohexane, there are no angle and torsional strains. Therefore, it is more stable than other cyclic compounds.
To be more precise, the bond angle is not 109.5°, even if it is in a chair conformation. However, all of the bond angles are close to 109.5°, and the bond angle strain of cyclohexane is low.
Equatorial, Axial, and the Concept of Ring Inversion
Equatorial and axial are important concepts in the stereochemistry of cyclohexane. In cyclohexane, there are 12 C-H bonds. They are as follows.
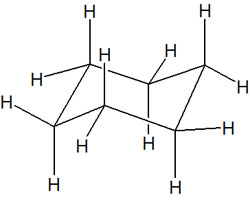
If we look at the C-H bond in the chair conformation, we can see that there are two types of hydrogen atoms; one in the horizontal position and the other in the vertical position. In cyclohexane, they are called, respectively, the following.
- Equatorial: horizontal position.
- Axial: vertical position.

Among the bonds in cyclohexane, the equatorial is located in a horizontal position. In the axial, on the other hand, the hydrogen atoms are located in the upward or downward position. It is important to understand that there are two types of positions in the chair conformation.
-Cyclohexane Cause Ring Inversion
Note that the chair conformation of cyclohexane is ring invertible. Therefore, it can be equatorial in some cases and axial under other conditions.
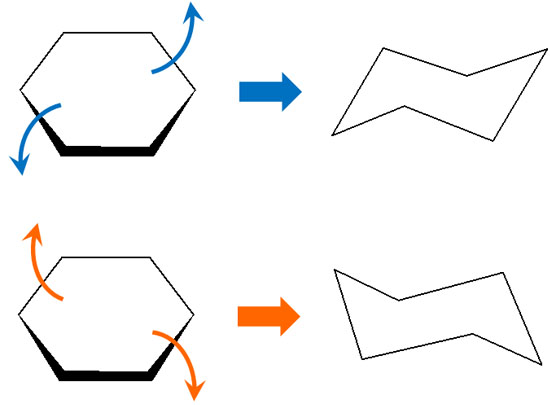
In organic chemistry, you have to think in terms of stereochemistry, so you have to take into account that in cyclohexane, there are ring inversions.
Steric Hindrance Due to 1,3-Diaxial Interactions (Repulsion)
How do we think about the stereochemistry of cyclohexane when it has a substituent? For example, suppose we have methylcyclohexane. If you write methylcyclohexane in a plane, you can write only one type of methylcyclohexane.
On the other hand, if you think about it in terms of stereochemistry, you’ll notice that cyclohexane has two conformations. The position depends on whether the methyl group is in the equatorial or the axial.
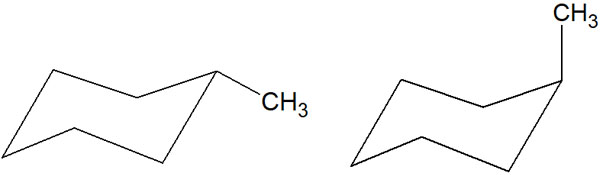
What is the best way to think about this point? When cyclohexane is in the chair conformation, is the methyl group in the equatorial or axial position?
The answer is that the methyl group of cyclohexane is more stable in the equatorial position. If it is in the axial position, it will be unstable due to its higher energy. The reason for this is the steric hindrance.
The presence of a methyl group in the axial position causes steric hindrance with the hydrogen atoms in the other axials. This causes steric strain and makes the molecule unstable. In the following, we describe how a methyl group causes repulsion.
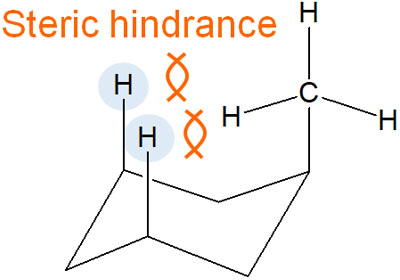
This steric strain at the axial position is called the 1,3-diaxial interaction; in the 1,3-diaxial interaction, it is repelled with two hydrogen atoms.
On the other hand, what about the equatorial position? In this case, there is no repulsion due to the 1,3-diaxial interaction. Due to the presence of the methyl group on the side of the cyclohexane, it does not come close to the other hydrogen atoms. It is as follows.
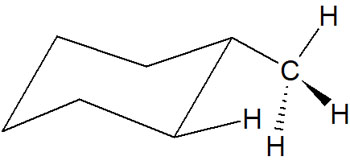
Because of the steric hindrance caused by 1,3-diaxial interactions, when cyclohexane has a substituent group, it often has an equatorial conformation.
In this article, we used methyl groups as an example of steric hindrance. Note that if a larger substituent, such as a tert-butyl group, is attached to the cyclohexane instead of a methyl group, the steric strain caused by the 1,3-diaxial interaction is greater. In this case, the substituent will try to become more equatorial conformation.
Stability of cis-trans in Disubstituted Cyclohexane
Cyclic compounds do not necessarily have only one substituent; they can have two substituents. In this case, how should we think about it?
When there are two substituents, there are two patterns of substitution: cis and trans. For example, if a compound has two methyl groups, you can write the following two structural formulas.
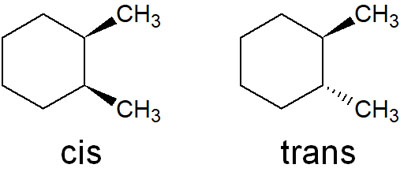
Because of these stereoisomers, both cis and trans have to be considered in the disubstituted cyclohexane.
Note that the following two conformations exist in cis.
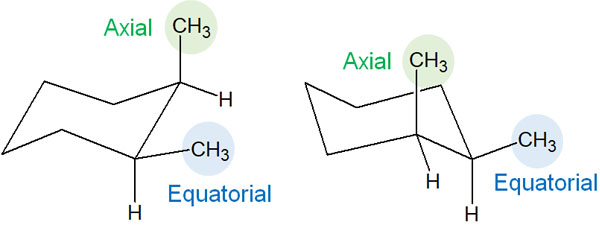
In cis, if one methyl group is in the equatorial position, the other methyl group is in the axial. Naturally, the methyl group in the axial causes a 1,3-diaxial interaction. However, in both cases, the methyl group is present in the axial, so there is no difference in energy for each of the conformations.
In contrast, what happens in trans? In trans, the two methyl groups are both in the equatorial or axial position.
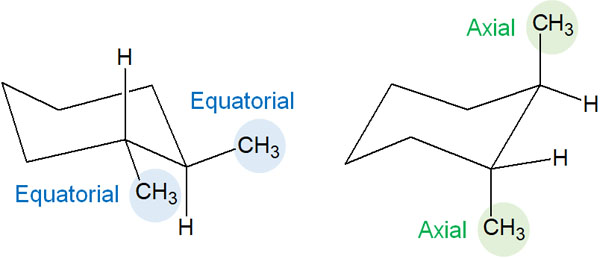
As already explained, the presence of a methyl group at the position of the axial leads to steric repulsion due to 1,3-diaxial interactions. Therefore, among the stereoisomers, the equatorial conformation is preferred in the trans disubstituted cyclohexane because of the difference in energy.
The Boat Conformation Is Highly Strained
We have explained the strains of cyclic compounds and the stability of the chair conformation in cyclohexane. The chair conformation is the most stable form of cyclohexane.
However, chair conformation is not the only form that cyclohexane can take. It is also possible for cyclohexane to be in other conformations. There are several types of conformations that can take cyclohexane. Among them, there is a boat conformation that is particularly important.
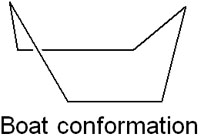
We have already mentioned that cyclohexane is capable of ring inversion. When ring inversion occurs, cyclohexane takes on a boat conformation as an intermediate.
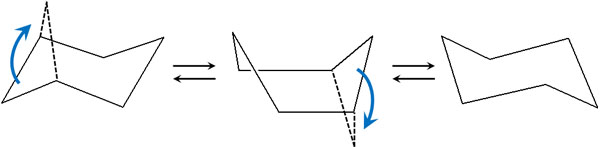
Compared to the chair conformation, the boat conformation has a higher strain. In a boat conformation, it causes torsion. Instead of 60°, the overlap causes the dihedral angle to be 0°. As a result, a torsional strain occurs in boat conformations.
If we write the boat conformation in Newman projection, it looks like the following.

In addition, the boat conformation has a steric hindrance. Because of the proximity of the hydrogen atoms, a steric strain occurs due to steric repulsion.
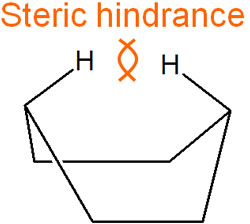
For these reasons, the potential energy of the boat conformation is high.
Steric Hindrances in the Energy Diagram of Ring Inversions
In order for cyclohexane to change from the chair conformation to the boat conformation, it must change its 3D conformation. As a cyclic compound, it is necessary to change its shape while its three-dimensional structure has already been fixed, so it requires a lot of energy to invert the ring.
Of course, a large amount of energy is required to change from a chair conformation to a boat conformation. A large amount of energy is also required to return from a boat conformation to a chair conformation.
When cyclohexane undergoes ring inversion, it must pass through several unstable structures. These structures include a twist-boat conformation. The energy diagram is shown below.
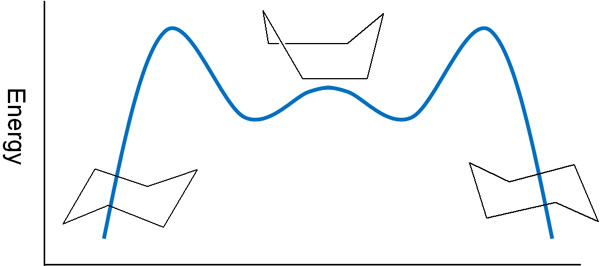
Even though ring inversion occurs in cyclohexane, it is necessary to change the conformation through these energies, such as the twist-boat conformation and the boat conformation. As an intermediate, it becomes the boat conformation, and then the conformation changes.
Stability Is Important in the Stereochemistry of Cyclic Compounds
In organic chemistry, we often draw compounds in a plane. However, when molecules react chemically, there are many situations that require us to consider stereochemistry. One of them is cyclic compounds. When we think of cyclic compounds in terms of three dimensions, the energy can be higher or lower by causing strain.
Also, in cyclohexane, the energy state differs depending on where the substituent is located in the chair conformation: equatorial or axial. For cis-trans isomers, the conformation also differs depending on the location of the substituent.
In addition to that, cyclohexane is ring invertible. When the ring is inverted, it becomes a boat conformation, and the energy state becomes higher due to steric repulsion and other strains.
In this way, the stability of cyclic compounds is considered in terms of three dimensions. Since the conformation is related to the reactivity of organic chemistry, it is important to understand when the structure is stable.





