There are many types of compounds that have aromatic properties. All compounds are aromatic if they have a benzene ring in the molecule.
However, there are aromatic compounds that are different from benzene rings. These are heterocyclic compounds. Aromatic heterocyclic compounds show aromaticity but have different properties from benzene rings.
What should we pay attention to when dealing with heterocyclic compounds in organic chemistry? And what is the nature of aromatic heterocyclic compounds?
One of the major areas of organic chemistry is heterocyclic compounds. We will explain how to think about the properties of heterocyclic compounds.
Table of Contents
What Are Aromatic Heterocyclic Compounds? Types of Heterocyclic Compounds
Before we understand heterocyclic compounds, we must learn about heteroatoms. Heteroatoms are atoms that are not carbon or hydrogen atoms. Typical heteroatoms include the following.
- Oxygen atoms (O)
- Nitrogen atom (N)
- Sulfur atom (S)
- Phosphorus atom (P)
Although there are many cyclic compounds, compounds with heteroatoms are called heterocyclic compounds. There are many heterocyclic compounds that show aromatic characters, such as the following compounds.
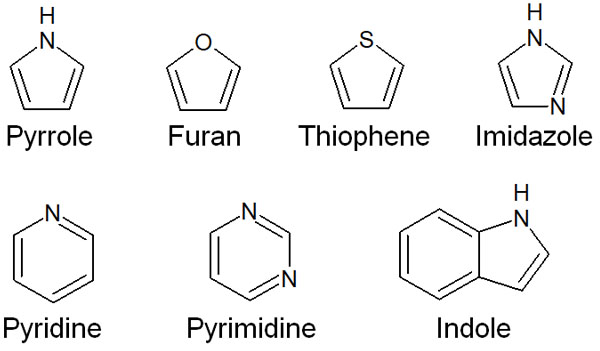
Of course, there are many other aromatic heterocyclic compounds.
A compound is aromatic if it has (4n+2) π-electrons. For all the above heterocyclic compounds, they have 6 or 10 π-electrons. Therefore, they are aromatic.
-There Are Many Types of Aliphatic Heterocyclic Compounds
For reference, there are many types of aliphatic heterocyclic compounds as well. If there are no double bonds to satisfy aromaticity, but there are heteroatoms in the ring compound, it is an aliphatic heterocyclic compound. For example, aliphatic heterocyclic compounds include the following.
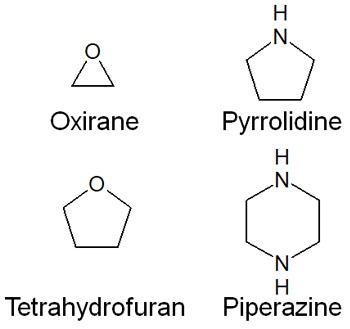
In any case, there are many types of heterocyclic compounds.
Aromatic and Basic Properties of Pyridine and Pyrrole
Even though they are the same aromatic heterocyclic compounds, the properties of the five- and six-membered rings are different. Pyridine and pyrrole are frequently used as examples of this.

For six-membered heterocyclic compounds, it is easy to understand that they have aromatic characters. Pyridine is a six-membered ring with three double bonds and six π-electrons, which makes it aromatic.
But why does pyrrole exhibit aromaticity? Pyrrole has only two double bonds. Therefore, you might think that pyrrole has only four π-electrons. If pyrrole has four π-electrons, it cannot show aromaticity.
-A Nitrogen Atom of Pyrrole is Involved in Aromaticity (2p Orbital)
In order to show aromaticity, there must be six π-electrons in the pyrrole. So if we check the nitrogen atom of the pyrrole, we can see that there is an unshared electron pair (lone pair). The nitrogen atom is bonded to two carbon atoms and one hydrogen atom. Not only that, but there are two electrons that are not involved in the bond.

In the case of pyrrole, the unshared electron pairs in the nitrogen atom are involved in aromaticity. In addition to the four π-electrons in the double bond, two electrons (lone pair) possessed by the nitrogen atom are involved in the aromaticity of pyrrole. This makes pyrrole aromatic.
Normally, when the nitrogen atom is present, it shows basicity. Therefore, the lone pair present in the nitrogen atom can nucleophilically attack other molecules.
However, the unshared electron pair of pyrrole exists in the 2p orbital (the orbital involved in aromaticity). The lone pair is not on a nitrogen atom but is distributed throughout the ring.
Because the electrons are delocalized (i.e., they are distributed in various places), the lone pair cannot attack other molecules, even though the nitrogen atom has an unshared electron pair. In other words, there is no nucleophilic (basic) nature, and pyrrole does not show basicity.
-The Lone Pair of Pyridine Is Involved in the sp2 Hybrid Orbital
In contrast, pyridine shows basicity. Because pyridine has three double bonds, the unshared electron pairs (lone pairs) in pyridine need not be involved in aromaticity. Therefore, the lone pair of the nitrogen atom in pyridine is not involved in the 2p orbital but in the sp2 hybrid orbital.
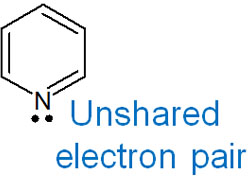
Since the lone pair is involved in the sp2 hybrid orbital and not in the aromaticity, the unshared electron pair on the nitrogen atom shows basicity.
Also, unlike pyrrole, which is insoluble in water, pyridine is hydrophilic. Pyridine is water soluble because it forms hydrogen bonds in the solution.
In the case of pyrrole, the lone pair of a nitrogen atom is involved in the aromaticity, and the nitrogen atom does not have the electrons to make hydrogen bonds. In pyridine, on the other hand, a nitrogen atom is negatively charged, allowing the lone pair to form hydrogen bonds with water molecules.
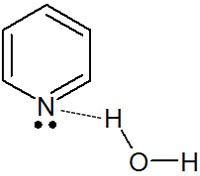
Therefore, pyridine is water soluble. Even though they are the same aromatic heterocyclic compounds, the properties of five- and six-membered rings are quite different.
Reactivity of Electrophilic Substitution Reactions Differ Between 5 and 6-Membered Rings
How does the reactivity in aromatic heterocyclic compounds differ? Due to the difference in properties, the reactivity of aromatic compounds also differs between 5-membered and 6-membered rings.
For heterocyclic compounds that show aromaticity, the following points should be checked.
- Reactivity of the compound: high or low electron density
- Where the reaction occurs: orientation
In aromatic compounds with a benzene ring, the electron density and orientation change depending on the nature of the substituent. Even in aromatic heterocyclic compounds, the reactivity changes depending on the substituents. However, what is more important is how the reactivity changes depending on the five- and six-membered rings.
The 5-Membered Ring Has a Higher Electron Density and a Substituent Will Bound to the 2nd Position
An important synthetic reaction in aromatic compounds is the electrophilic substitution reaction. Electrophilic aromatic substitution is a reaction in which a hydrogen atom on a heterocyclic ring is replaced by a substituent.
When the electron density of the aromatic ring is high, the electrophilic substitution reaction is more likely to occur. This is because the higher the electron density of the aromatic ring, the easier it is to attack the electrophiles.
In 5-membered heterocyclic compounds, the electron density of the aromatic ring is high because the aromatic ring has six π-electrons, even though it is a five-membered ring. Since there are six π-electrons in the 5-membered ring, it can be calculated as follows.
- 6 (π electrons) ÷ 5-membered ring = 1.2
One carbon atom has 1.2 pi-electrons in it. This is the reason why 5-membered ring heterocyclic compounds are highly reactive.
-The Orientation Is 2nd (α-Position)
For a 5-membered heterocyclic compound, there are two reaction sites: the 2nd (α-position) and the 3rd (β-position). At which position does the electrophilic aromatic substitution reaction take place?
The answer is that the 2nd position is where the electrophilic substitution reaction takes place. When an electrophilic agent reacts with an aromatic ring, more resonant structures can be written if there is a substituent at the 2-position. The reaction mechanism is as follows.
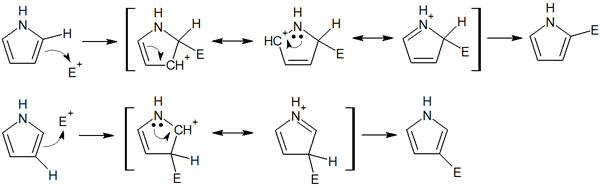
Since the resonant structures can be written, the electrons can be dispersed in many places, and the structure is more stable. For this reason, electrophilic substitution reactions with 5-membered heterocyclic compounds occur in the 2nd position (α-position).
6-Membered Rings Have Low Electron Density and Are meta-Oriented
On the other hand, what about the heterocyclic compounds with six-membered rings? Unlike the 5-membered heterocyclic compounds, the electron density is lower in the 6-membered ring. There are fewer electrons in the aromatic carbon atoms.
When we write the resonance structure, the double bond (electron) in the aromatic ring can be transferred to the nitrogen atom. This results in the following resonance structure.
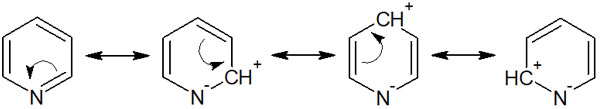
Thus, the carbon atom is positively charged. As a result of the transfer of electrons from the aromatic ring to the nitrogen atom, the electron density of the aromatic ring is lowered, making it less reactive. In fact, pyridines must be subjected to extreme conditions in order to undergo an electrophilic substitution reaction.
In addition, by checking the resonance structure, we can see that the ortho and para positions are positively charged. Therefore, for six-membered heterocyclic compounds, such as pyridine, an electrophilic substitution always takes place at the meta-position. Because of the low electron density in the ortho and para positions, the reaction occurs in the meta position.
The reason for the meta-orientation in pyridines is that the carbon atom is not positively charged only in the meta-position.
Nucleophilic Aromatic Substitution Reactions with Pyridine
The reactions that occur in aromatic heterocyclic compounds are not limited to electrophilic substitution reactions. Nucleophilic aromatic substitution reactions also occur. Nucleophilic substitution reactions are reactions in which a nucleophile attacks and replaces a substituent on a benzene ring.
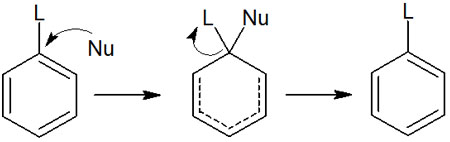
Aromatic compounds do not normally undergo nucleophilic substitution. Exceptionally, aromatic compounds with low electron density, such as pyridine, can undergo nucleophilic aromatic substitution reactions.
Specifically, nucleophilic substitution occurs when a halogen such as fluorine (F) or chlorine (Cl) is bonded to the pyridine in ortho or para.
As mentioned above, six-membered aromatic heterocyclic compounds have positive charges at the ortho and para positions. This makes it easier to attack by negatively charged nucleophiles. If these conditions are met, a nucleophilic substitution reaction to a halogen occurs.
Learn Reactivity and Orientation of Heterocyclic Compounds
One major genre in organic chemistry synthesis is heterocyclic compounds. There are many compounds that have heterocyclic rings in the molecule, and you have to learn how they react chemically.
However, there are some major differences in heterocyclic compounds compared to benzene rings. Also, even though the compounds exhibit aromaticity, the characteristics of a 5-membered ring and a 6-membered ring are quite different. They have different properties such as basicity, reactivity, and orientation.
There are many types of aromatic heterocyclic compounds, not only 5-membered rings and 6-membered rings, but also more complex ring structures are common. There are also many compounds that have more than one heteroatom in the molecule. So, we have used simple heterocyclic compounds such as pyrrole and pyridine as examples.
Because they are aromatic compounds, the compounds can undergo electrophilic and nucleophilic substitution reactions. There are aromatic specific reactivities and orientations that are unique to heteroatoms, so make sure you understand these properties.