There are many compounds that have ketones and aldehydes as functional groups. They are also known as carbonyl and formyl groups and are important functional groups because they cause many types of chemical reactions.
Ketones and aldehydes are highly reactive, so if no measures are taken, the carbonyl carbon will undergo nucleophilic attack, and the synthetic reaction will proceed. Therefore, the carbonyl and formyl groups must be protected to prevent them from reacting. These are called protecting groups (or protective groups).
Acetals are frequently used as protecting groups for carbonyl compounds. We can synthesize an acetal from a carbonyl compound via a hemiacetal.
Why are these protective groups useful? And what are the reaction mechanisms and deprotection methods? These will be explained in this section.
Table of Contents
Ketones and Aldehydes Hydrate to Form Hemiacetal
Some compounds have different forms when they exist in solid and when they are dissolved in solution. Typical examples of this are compounds with carbonyl or formyl groups.
Compounds with ketones and aldehydes have a different form when dissolved in water or alcohol solvents. This is called hydration. In the case of aldehydes, in particular, most compounds change their shape through hydration reactions.
Ketones and aldehydes undergo hydration reactions, and the resulting compounds are hemiacetals. The reaction mechanism of hemiacetal is as follows.
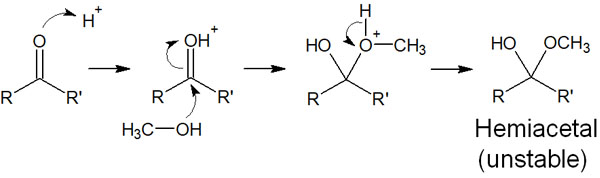
The above figure shows the reaction mechanism in acidic conditions. Hemiacetals are formed not only in acidic conditions but also in basic conditions. For ketones and aldehydes, hemiacetals are formed in many cases.
Hemiacetal Is Unstable; Hydrates Are Reversible Reactions
However, it is not possible to isolate hemiacetals when doing experiments in organic chemistry. There is no doubt that it will hydrate and react when compounds with carbonyl and formyl groups are dissolved. However, since hemiacetals are unstable, they return to their original ketone or aldehyde compounds when water or alcohol is removed.
This is because the hydrate is a reversible reaction. Even though hemiacetal is formed by the hydration reaction, when water or alcohol is no longer present, the reverse reaction occurs.
The reaction mechanism by which the hydrate returns to the original carbonyl compound is as follows.
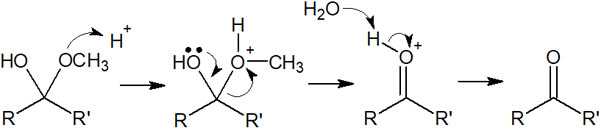
Even if the compound is a hemiacetal in an aqueous solution, removing the solvent will not yield a hemiacetal compound because the reversible reaction will restore it.
Acetal Can Be Synthesized from Hemiacetal
In addition, if the reaction is carried out under acidic conditions, the reaction proceeds further from hemiacetal and yields acetal. Under neutral or basic conditions, acetal is not synthesized. Acetals are obtained only under acidic conditions.
When reacted under acidic conditions, hemiacetal is converted to acetal by the following reaction mechanism.
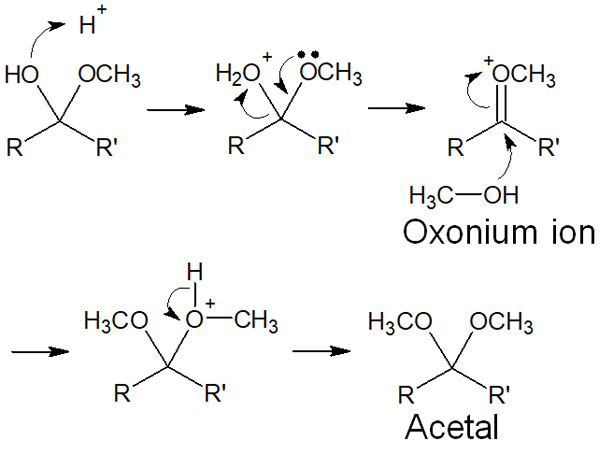
An important part of this reaction is that the reaction produces water (H2O). Initially, the H2O is released when an oxygen atom bonds with a proton. This results in the formation of an oxonium ion.
Without the presence of a proton (H+) in the solution, the synthesis of acetal will not proceed. This is the reason why acetal cannot be obtained in neutral or basic conditions.
Oxonium ions are unstable intermediates. Therefore, the reaction proceeds further when the alcohol attacks them, resulting in the formation of acetal. Unlike hemiacetal, acetal is a stable substance and can be isolated.
The Synthesis Reaction Proceeds While Removing Water
Just as hemiacetal is a reversible reaction, acetal is also reversible. When water is added to the acetal synthesized by the hydration reaction, it returns to the ketone or aldehyde.
However, as mentioned earlier in the reaction mechanism, water is produced when acetal is synthesized from hemiacetal. In the presence of water, a reversible reaction occurs, and the acetal is hydrolyzed and returns to its original state. Therefore, when synthesizing acetals, it is necessary to proceed with the synthetic reaction while removing the water produced.
-Hydrolysis Occurs Under Acidic Conditions; Acetals Are Stable Under Basic Conditions
The reaction conditions for the hydrolysis of acetal to return to the original carbonyl compound are acidic. If the reaction conditions are not acidic, the hydrolysis of the acetal will not occur.
Under basic conditions, acetal is stable. It is important to understand that acetals are hydrolyzed by acids but are stable to bases.
Use of Acetal as a Protective Group
Why is acetalization so important in organic chemistry? It’s because acetal is frequently used as a protecting group for ketones and aldehydes.
Carbonyl and formyl groups are highly reactive. Nucleophilic addition reactions can easily take place when the carbonyl carbon is attacked by nucleophiles. For example, a Grignard reagent is a very strong basic compound. If there is a ketone in the molecule, it will react intramolecularly as follows.
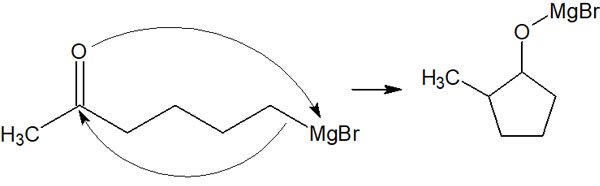
So, we will protect the acetal in advance. If you convert the ketone to acetal, you can add magnesium (Mg) to make the Grignard reagent, and it will not cause an intramolecular reaction.
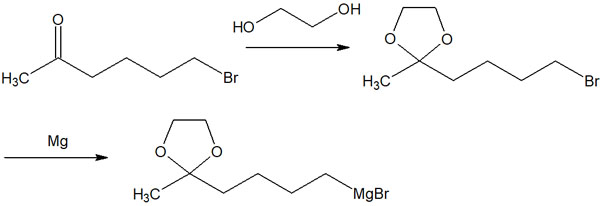
As mentioned above, acetals are stable in basic conditions. Since Grignard reagents are strong bases, they do not react with acetals. These protective groups allow for regioselective organic synthesis.
-Cyclic Acetals Are More Stable Than Chain Acetals and Are Used More Frequently
For reference, cyclic acetals are more stable than chain acetals. For this reason, ethylene glycol is often used to protect acetals.
When ethylene glycol is used, a five-membered cyclic acetal is formed. This compound is called dioxolane.
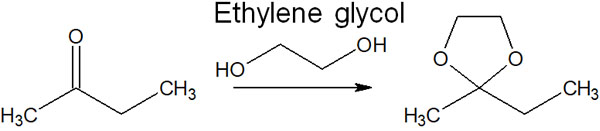
Ethylene glycol is one of the cheapest organic compounds you can get. So acetal protecting groups to carbonyl compounds can protect them from nucleophilic attack by basic substances.
Deprotection by Hydrolysis to Obtain Target Compounds
Naturally, the protective group of the acetal must be removed at some point. The removal of the protective group is called deprotection.
The method for deprotection is simple. All you have to do is mix it with an acidic solution such as hydrochloric acid or sulfuric acid. The addition of these acid catalysts is enough to cause hydrolysis, which can be easily converted back to ketone or aldehyde.
The reaction mechanism of hydrolysis of acetal-protecting compounds to carbonyl compounds has already been explained, so we will skip the details. The reaction mechanism of hemiacetal hydrolysis to ketone or aldehyde has been described earlier. By the same reaction mechanism, carbonyl compounds can be obtained.
Acetal protecting groups are frequently used in organic chemistry because they can easily protect carbonyl and formyl groups, and the deprotection method is simple.
There Are Many Applications of Acetal Protecting Groups, Such as Alcohol Protecting
Protecting groups are frequently used in many situations. Acetal protection is used not only for carbonyl compounds but also for the protection of hydroxy groups (-OH).
For example, suppose we have a compound of 1,3 diols; by reacting the 1,3 diols with a carbonyl compound, we can synthesize a cyclic acetal. This will result in a protective group for the 1,3 diols.
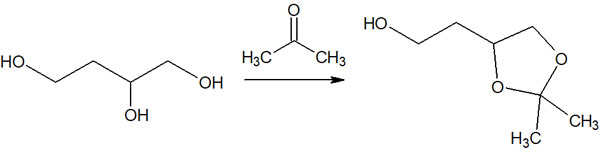
-Alcohol Protecting Using THP and MOM Groups
On the other hand, it is possible to protect not only 1,3 diols but also one hydroxy group. The following are examples of protecting groups.
- THP (tetrahydropyranyl)
- MOM (Monomethoxymethyl)
When making THP, DHP (dihydropyran) is used as a reagent. On the other hand, for the MOM group, chloromethyl methyl ether is used as a reagent. It is as follows.
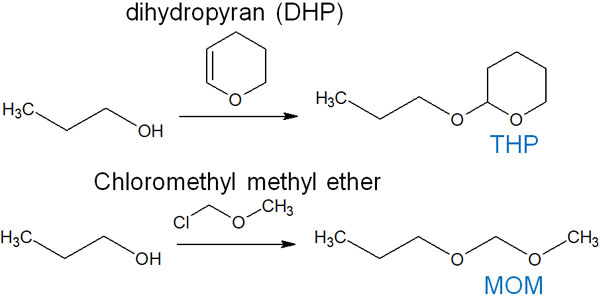
It is important to note that both THP and MOM are acetal. We have discussed acetal protecting groups in carbonyl compounds, and we can also protect alcohols by using the THP and MOM groups.
Acetals are stable in basic conditions, so if the alcohol is protected and then reacted under basic conditions, no side reactions will occur. Acetal protection is widely used not only for carbonyl and formyl groups but also for hydroxy groups.
Learn the Properties of Acetal, an Important Protecting Group
Carbonyl compounds undergo many types of synthetic reactions. One of the most important reactions is hydration. Carbonyl compounds react with water or alcohol to produce hemiacetal. Acetal can also be obtained under acidic conditions.
The reason why acetal synthesis is so important is that it is a protective group. Protecting groups are useful if you want to react in a regioselective manner, only at a specific position.
In addition to carbonyl and formyl groups, acetals are also useful as protecting groups for alcohols. Acetal protecting groups are beneficial in many situations.
Acetals are frequently used as protective groups because they can easily be deprotected under acidic conditions. So, let’s make sure you understand the properties of acetals.