It is important to analyze the structure of chemical compounds to find out what compounds are present in the solution. By analyzing the structure of these compounds, it is possible to investigate the molecules and proteins.
HPLC (high performance liquid chromatography) and MS (mass spectrometry) are frequently used as methods to investigate what substances are contained in a solution. Importantly, a combination of these methods is widely used.
LC/MS, MS/MS, and LC/MS/MS are examples of these methods. To understand the difference between these analytical methods, let’s understand that they are a combination of HPLC and MS.
So, what principles are used to examine substances in solution? Let’s take a look at the differences and principles of each analytical method.
Table of Contents
HPLC and MS (Mass Spectrometry) Frequently Used in Analytical Instruments
HPLC and MS are the most frequently used analytical instruments in many laboratories. They are used so frequently that it is difficult to find a laboratory that does not use them, including organic chemistry and biochemistry. HPLC and MS are used in almost every field, including medicine, food, and the environment.
HPLC is a type of chromatography. It is difficult to analyze a mixture of several substances in the solution. If it includes multiple compounds, the data will contain a lot of noise due to the influence of other compounds, making it impossible to analyze the data correctly.
Chromatography is a method to separate multiple substances into a single compound. HPLC is the most widely used chromatography method.
After separating a substance by chromatography, it is necessary to determine the nature of the compound. MS is used to determine the molecular weight, which allows us to investigate the weight of the target compound. The combination of HPLC and MS is called LC/MS.
High Performance Liquid Chromatography (HPLC) Is the Most Common Chromatography
Why is it possible to separate substances in HPLC? One reason is that it uses a substance that adsorbs compounds as a stationary phase.
High performance liquid chromatography is also known as reversed-phase chromatography. Reversed-phase chromatography utilizes a highly hydrophobic substance as the stationary phase. This is called an ODS column. Think of an ODS column as being packed with a highly hydrophobic substance.
Water and oil do not mix. However, substances with the same properties will strongly attract each other. For example, a compound with high fat-solubility will strongly adsorb to the ODS column, which is also highly hydrophobic. On the other hand, highly polar compounds are less likely to be adsorbed on an ODS column and will pass quickly with the liquid (mobile phase).
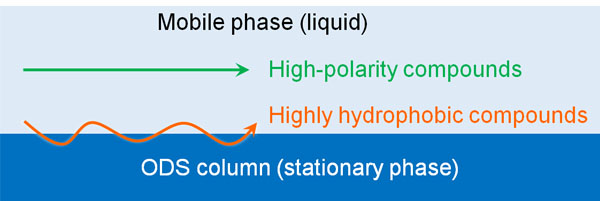
The more hydrophobic a compound is, the more it will gradually move forward while being adsorbed by the ODS column. In other words, the speed at which a compound moves through the ODS column (stationary phase) will vary depending on the compound.
In HPLC, the organic solvent (mobile phase) will continue to flow. As a result of the compounds moving at different speeds, we can separate the substances as follows.
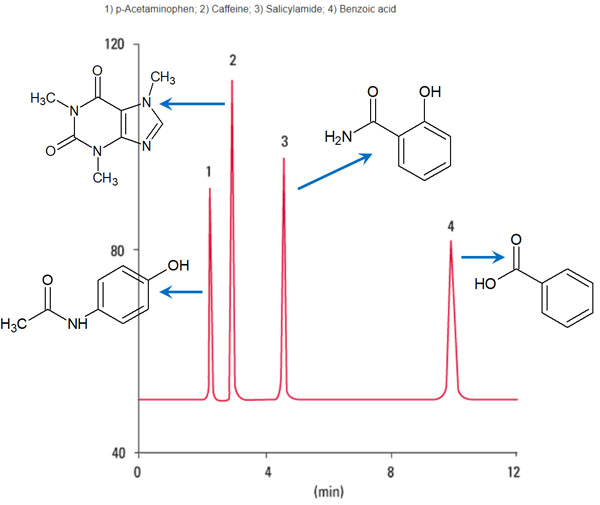
Source: Partial modification of the TOSOH database
High performance liquid chromatography has these characteristics.
Determine the Molecular Weight of the Compound by Mass Spectrometry (MS)
An entirely different analytical method than HPLC is mass spectrometry; think of MS as measuring the molecular weight of a target compound. MS is a device that analyzes the mass of a molecule after it has been ionized.
In mass spectrometry, positive ions are created. When a magnetic field is applied to the ionized material, it curves. The degree of the curve differs depending on the molecular weight of the molecule.
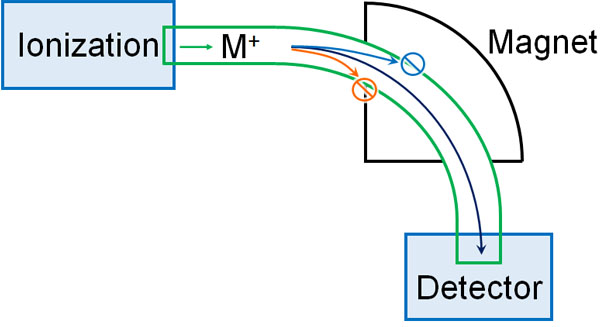
Then, we change the strength of the magnetic field little by little. When the detector measures the molecule, the peak will appear. This is called a mass spectrum.
For example, when acetic acid is measured by mass spectrometry, the following mass spectrum is obtained.
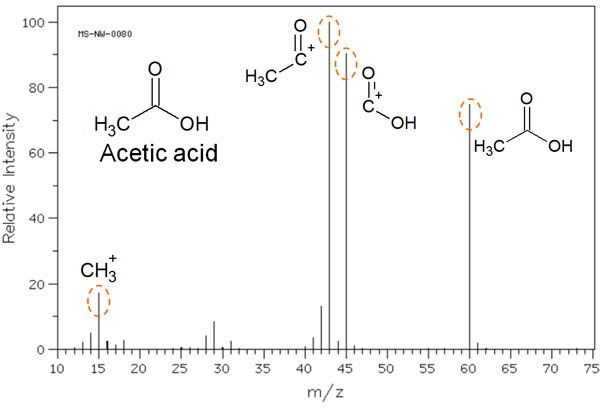
In the mass spectrum, the molecular weight peak of acetic acid can be observed. However, in MS, high energy ions are bombarded, resulting in radical cations.
Radical cations are highly reactive and can cause the bonds in the molecule to cleave. As a result, not only the molecular weight of acetic acid, but also the molecules after the cleavage of the acetate structure can be observed in the mass spectrum. These cleaved structures are called fragment ions.
LC/MS Is a Combination of HPLC and MS
HPLC and MS are completely different in nature, but LC/MS (liquid chromatography mass spectrometry) is a combination of both. Let’s understand that LC/MS is an instrument that can perform both HPLC and MS at the same time.
When analyzing by MS, substances must be separated by chromatography such as HPLC in advance. Even if the molecular weight is measured while multiple substances are mixed, it is unclear whether the peak that appears is the target compound peak or not.
However, after separating the substances by HPLC, it is time-consuming to analyze all substances by mass. In order to save time, the molecular weight is measured by MS immediately after the separation of the compounds by HPLC. As a researcher, it is very convenient to perform both separations by HPLC and molecular weight measurement at the same time.
-Single Molecule Peaks Are Observed in LC/MS Mass Spectrometry
Earlier, we discussed mass spectra using general mass spectrometry methods. Electron ionization (EI) is frequently used in mass spectrometry. The compound is ionized by bombarding it with high-energy electrons. This causes multiple molecules, including fragment ions, to be measured.
However, there are a number of ways to use MS. For mass spectrometers used in LC/MS, think of it as observing only the molecular weight of the target compound, without producing fragment ions.
For example, acetic acid, with a molecular weight of 60, will only show a peak at 60m/z (molecular weight 60) when measured by LC/MS. LC/MS does not break molecular bonds by creating radical cations. This means that multiple fragment ions cannot be produced.
While there are various methods of MS, the mass spectrometry used in LC/MS measure molecular weight in a way that does not produce fragment ions.
MS/MS Measures the Molecular Weight and Fragment Ions of a Compound
LC/MS/MS is also frequently used in analytical instruments; LC/MS is a combination of HPLC and MS, as I explained earlier. In contrast, what is LC/MS/MS?
In order to understand the concepts and characteristics of LC/MS/MS, you must learn about MS/MS first.
In the most common mass spectrometry methods, multiple peaks are observed in the mass spectrum. This is because the production of radical cations results in the cleavage of bonds in the molecule, producing multiple fragment ions. However, we do not know which peak is the molecular weight of the original compound.
In the mass spectra we mentioned earlier, we gave the example of acetic acid. Because we measure a known compound called acetic acid, we already know its molecular weight.
However, what happens when we perform MS without knowing that the substance we are measuring is acetic acid? In this case, it is impossible to distinguish which peak in the mass spectrum is the molecular weight of the original compound and which is the peak of the fragment ion.
To solve this problem, a technique called MS/MS is used. In MS/MS, think of it as literally two mass spectrometers attached together.
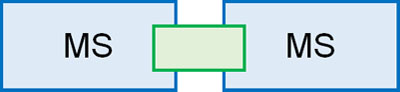
In the first MS, only the molecular weight of the target compound is measured. When measuring a single compound, only one peak is observed.
However, knowing the molecular weight alone is not enough to determine the structure of an unknown compound. Therefore, fragment ions are created by applying high energy to the molecule. By intermolecular cleavage, multiple molecules are generated.
In a collision cell, the compound is bombarded by an inert gas. The resulting collision energy causes the molecules to dissociate into ions. In the second MS, we observe the fragment ions that are produced.

In materials with unpaired electrons, such as oxygen and nitrogen, the collisional energy tends to break the bonds and produce fragment ions. If we know the molecular weight of the fragment ion, we can infer the bonding within the molecule.
MS/MS is a technique that measures the molecular weight of the original compound, and then observes what kind of fragment ions are produced by the second MS.
Perform All Analyses Simultaneously with LC/MS/MS
Once you understand what we’ve discussed, it’s easy to understand what an LC/MS/MS instrument is: LC/MS/MS is a combination of HPLC and MS/MS.
Analytical instruments are useless when multiple compounds are mixed together. Therefore, the compounds are separated by HPLC.
The compounds are then measured by the first MS at the exit of the HPLC. Mass spectrometry is used to determine the molecular weight of the compound. The bonds of the molecule are then cleaved and the fragment ions are observed by the second MS.
All of this can be done simultaneously with LC/MS/MS. LC/MS/MS can separate compounds, determine their molecular weight, and observe fragment ions.
Proteins Can Be Identified After Isolating to Peptides
If you want to do LC/MC/MS on small organic compounds, this is all you need to know. This knowledge is sufficient for the analysis of small organic compounds, including food and environmental substances.
On the other hand, there is more to consider, especially in the study of biochemistry. This is because these scientific experiments frequently involve the use of proteins.
If you run a protein through HPLC without treating it, you won’t be able to measure it. Proteins are macromolecules that can clog the column.
Therefore, in LC/MC/MS using proteins, the proteins are broken down into small pieces in advance. Proteases are known to be enzymes that break down proteins. Usually, trypsin is used as a protease. By adding trypsin, high molecular weight proteins are cleaved.
When the protein is cleaved, it becomes peptides. Peptides are made up of a series of amino acids. Peptides are able to pass through HPLC columns. Therefore, we analyze peptides by LC/MC/MS.
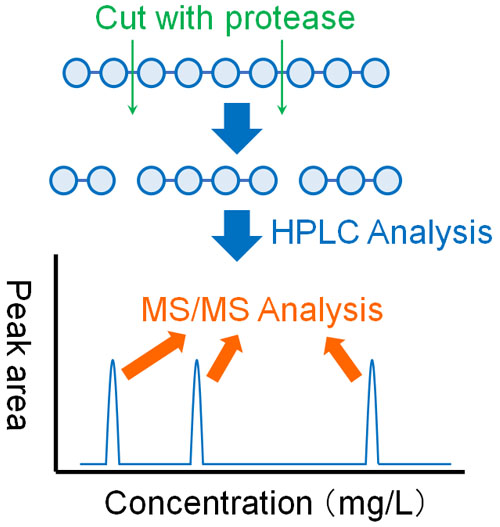
Peptides cleaved by proteases (trypsin) can be analyzed by LC/MC/MS to obtain a number of MS/MS data. Then, analyze the MS/MS data to see what peptides were detected.
Different proteins have different cleavage points by proteases. In other words, there are different peptides produced. By comparing the observed data with the known data (protein database), we can identify which protein is present.
It doesn’t matter how many proteins are mixed together; each peptide can be separated by HPLC, and then analyzed by MS/MS to identify the present proteins.
However, not all proteins in the solution can be identified. It is also not possible to examine minor compounds that are not present in the database. Although the advantages of LC/MC/MS are great, it is important to understand that it has these disadvantages.
The study of protein structure is called proteomics (proteomic analysis). The use of LC/MC/MS is indispensable for proteomics (proteomic analysis).
LC/MS/MS Techniques Important in Chemical Analysis
In scientific experiments, we often do chromatography and mass spectrometry. However, it takes a lot of time to perform each technique one by one. It would be more efficient if multiple data could be obtained by a single experiment.
Therefore, LC/MS is a combination of HPLC and MS. In addition, LC/MS/MS is also used to observe fragment ions. Compared to LC/MS, LC/MS/MS is more sophisticated.
In many cases, multiple substances are mixed together and cannot be chemically analyzed as they are. Therefore, HPLC is used to separate the compounds, and MS/MS is used to check the details of the contained compounds.
These methods can be used to detect unknown compounds. Proteomics can also be used to identify proteins. The most frequently used analytical instruments are LC/MS and LC/MS/MS.