There are many organic compounds that involve oxygen atoms. Among these compounds, ethers are the functional groups in which carbon atoms are connected by oxygen atoms.
There are only a few types of important organic reactions using ethers, and all you have to do is understand the cleavage reaction under acidic conditions. In other words, one molecule is split into two by cleaving of the ether.
Also, the ether of a triangular cyclic compound is called an epoxide. Epoxides are important compounds in organic chemistry, and we need to understand how to synthesize epoxides and how to subsequently react them to obtain the target compounds.
Therefore, we will explain the synthetic reactions of ethers and epoxides, and what products can be obtained.
Table of Contents
The Ether Is Cleaved by Acid Catalyst
There are functional groups that are difficult to chemically react with in their original state due to their low reactivity. One such group is the ether. The oxygen atom is strongly bonded to the carbon atom, and the bond will not cleave. Therefore, in order for the ether to chemically react, we have to activate it.
How can the ether be activated? One way to do this is through the use of an acid catalyst. By adding an acid catalyst such as sulfuric acid (H2SO4), hydrogen chloride (HCl), or hydrogen bromide (HBr), we can activate the ether.
Why does the presence of an acid catalyst activate ether? The presence of acid causes a hydrogen atom (proton) to bond to the oxygen atom of the ether; as a result of the H+ bonding, the ether becomes positively charged.
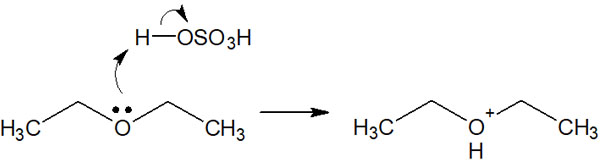
The positively charged oxygen atom becomes an excellent leaving group. Therefore, the ether cleavage causes it to split into two molecules.
Regioselectivity Is Based on the Stability of the Carbocation
When acid-catalyzed ether cleavage occurs, it seems that several different types of compounds can be obtained. For example, the following two compounds seem to be produced as intermediates under strong acid conditions when reacting with hydrogen bromide.
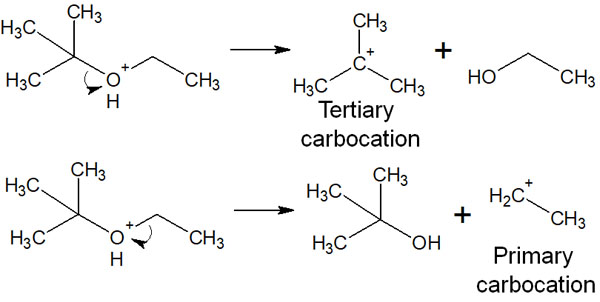
However, in the actual synthetic reaction, only one intermediate is produced. Therefore, only a specific compound can be synthesized instead of several compounds.
Why is there only one intermediate produced? This is because of the stability of the intermediate, the carbocation. The stability of the carbocation is shown below.
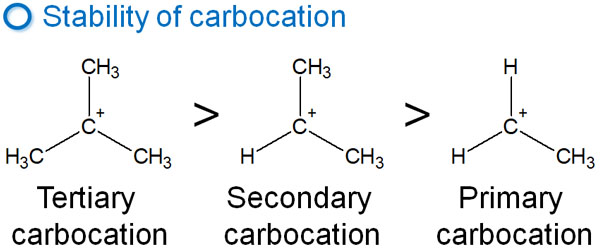
In the case of the previous compound, the tertiary or primary carbocation is formed after the ether is activated by the acid. However, since tertiary carbocation is more stable, the tertiary carbocation is preferentially formed.
As a result, only a certain compound can be synthesized.
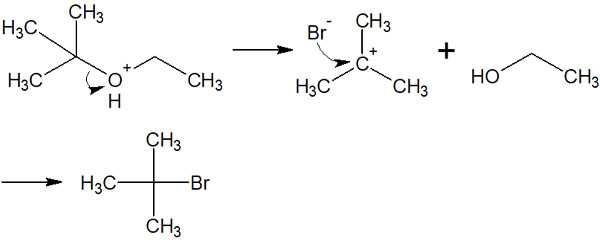
In ether cleavage reactions, there is regioselectivity. When hydrogen halides, such as hydrogen chloride (HCl) or hydrogen bromide (HBr), are used as acid catalysts, the location where ether cleavage occurs is determined.
-When the SN1 Reaction Is Impossible, the SN2 Reaction Occurs
The synthetic reaction described above is a nucleophilic substitution reaction, which is called the SN1 reaction. In the SN1 reaction, a carbocation intermediate is formed first, and then a nucleophile (e.g., bromide ion) attacks it to produce the compound.
What happens in the case of unstable intermediates such as primary carbocations, which are rarely formed under normal conditions? In this case, the synthetic reaction proceeds by the SN2 reaction, not the SN1 reaction in which the carbocation is formed as an intermediate.
The SN2 reaction is the one that gives the product in a single reaction without the formation of a carbocation. For example, we have the following.
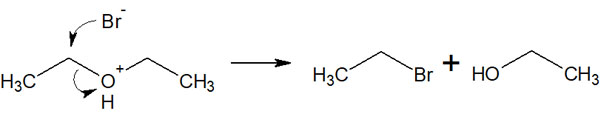
In any case, understand that the SN1 or SN2 reaction causes ether cleavage and splits one molecule into two.
Sulfuric Acid Catalysis Produces Alkenes in the E1 Reaction
When hydrogen halide is used as an acid catalyst, alkyl halides can be synthesized by ether cleavage, as described above. On the other hand, when sulfuric acid is used as an acid catalyst, alkenes (compounds with double bonds) can be synthesized.
In the case of hydrogen chloride (HCl) and hydrogen bromide (HBr), chloride or bromide ions are produced after providing hydrogen atoms (protons). These chloride or bromide ions attack the carbon atoms to produce alkyl halides.
In the case of sulfuric acid, on the other hand, even if H+ is provided, halogen ions are not generated. In addition, sulfate ions are stable and do not attack as nucleophiles.
Therefore, when sulfuric acid is used as a catalyst, the E1 reaction occurs. The E1 reaction is known as an elimination reaction, in which a compound with a double bond is formed when the sulfate ion pulls out a hydrogen atom (proton).
For example, in the case of the previous compound, the following compound can be synthesized by using sulfuric acid instead of hydrogen bromide.
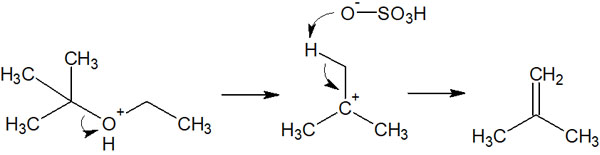
Understand that sulfuric acid cannot act as a nucleophilic reagent, so instead, it pulls out protons and causes an elimination reaction as an E1 reaction.
Synthesis of Epoxides by Epoxidation Reaction with Oxidizing Agents
As we have discussed, the acidic conditions allow the ether to cleave to yield other compounds. On the other hand, there is a special ether: epoxide. It is a cyclic ether and has a triangular shape.
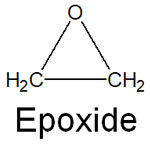
When learning about the reactivity of ethers, epoxides have a special organic reaction.
Triangular compounds have significant strain. They have a bonding angle of 60° and also have a torsional strain. In a triangular compound, it looks like the following.
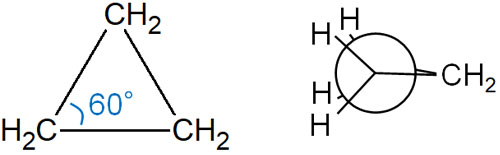
Therefore, triangular cyclic compounds are known to be highly reactive compounds.
-Oxidation of Alkenes to Obtain Epoxides
How do we synthesize epoxides? The synthetic reaction to obtain epoxides is called the epoxidation reaction. In epoxidation, alkenes are used.
Peracids are also used in epoxidation. The famous peracid is hydrogen peroxide, and epoxides can be synthesized by using peracids. The reaction mechanism is as follows.
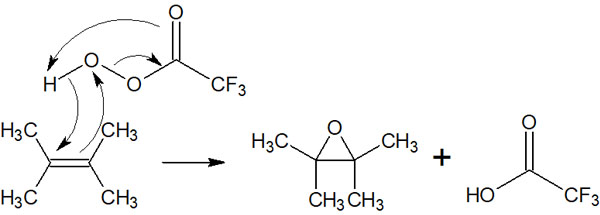
The reaction mechanism is a little more complicated, and the synthetic reaction by epoxidation occurs when electrons are transferred at once.
By syn-addition, the alkene reacts with the peracid, resulting in epoxidation. The synthetic reaction proceeds from the same side by syn-addition. Therefore, in cis compounds, epoxides of cis compounds can be obtained. In trans compounds, on the other hand, epoxides of trans compounds can be synthesized.
In epoxidation reaction by peracids, the synthetic reaction proceeds with the three-dimensional structure of the alkene preserved by syn-addition.
Reactivity of Epoxides under Acidic and Basic Conditions
After synthesizing epoxides, we will perform the next synthetic reaction. Epoxides are unstable compounds with high strain. Therefore, it is common to proceed with the next synthetic reaction using epoxide.
Because epoxides are highly reactive, there are so many nucleophiles that can be used.
- Alcohol (R-OH)
- Amine (R-NH2)
- Thiol (R-SH)
In addition to these nucleophiles, many other nucleophiles react with epoxides to obtain products. However, it is important to note that when a nucleophile attacks an epoxide, it can attack in two places.
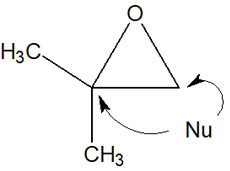
Which carbon atom does the nucleophilic agent attack? This depends on whether the reaction conditions are basic or acidic.
Nucleophilic Attack on a Carbon with Few Substituents under Basic Conditions
When reacting epoxides with reagents, the carbon atoms with fewer substituents are attacked in the synthetic reaction under basic conditions. In other words, there is regioselectivity in the synthetic reaction using epoxides.
The synthetic reaction proceeds as follows.
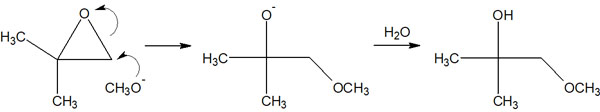
Why do nucleophiles attack carbon atoms with few substituents? It’s because of a steric hindrance. If there are many substituents, the nucleophile cannot approach the carbon atom due to steric hindrance. Therefore, it selectively attacks carbon atoms that have few substituents.
By attacking the carbon atom with less steric hindrance, only a specific product can be obtained. This is the synthetic reaction of epoxides under basic conditions.
Polysubstituted Carbons are Attacked in Acidic Conditions
On the other hand, how does it work under acidic conditions? In contrast to the basic condition, in the acidic condition, polysubstituted carbon atoms are attacked. Why are carbon atoms with large steric hindrances attacked by nucleophiles? The reason is that protons bind to oxygen atoms in the acidic reaction.
As already explained in the case of ether cleavage, the addition of an acid catalyst allows H+ to bond to the oxygen atom and activate the ether. In the same way, if it is acidic, a proton is attached to the oxygen atom of the epoxide, and the oxygen atom is positively charged.
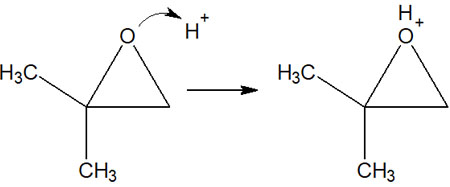
In addition, focusing on the carbon atom, if the bond is broken to produce a carbocation, one is a tertiary carbocation, and the other is a primary carbocation.
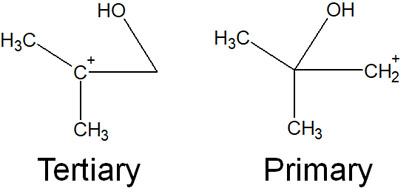
Considering the order of stability of the carbocations, the tertiary carbocations have more positive properties. Therefore, positively charged oxygen atoms and polysubstituted carbon atoms repel each other. The image is as follows.
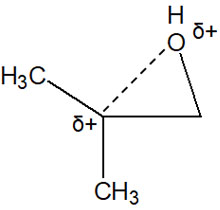
Compared to other bonds, the C-O bond is longer. The bond is also weaker than other bonds. As a result, the nucleophile attacks the polysubstituted carbon atoms.
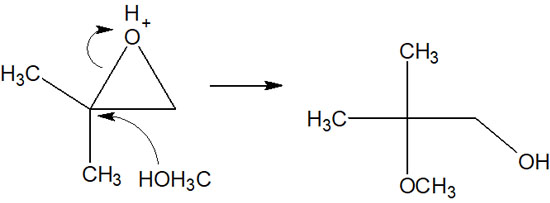
For these reasons, under acidic conditions, the nucleophile attacks carbon atoms with many substituents.
The compounds produced depend on whether the reaction conditions for the epoxide are acidic or basic. Try to understand these reaction mechanisms.
Synthetic Reactions of Ethers and Epoxides
For molecules with oxygen atoms, there are many ether compounds. So you have to learn how the ether is involved in organic chemistry.
The reactivity of the ether is poor. Therefore, it needs to be activated, and by using an acid catalyst, a proton is bonded to the oxygen atom, causing ether cleavage. Also, by changing the acid catalyst used, nucleophilic substitution reactions or E1 reactions (elimination reactions) will occur.
In addition, be sure to learn about the reactivity of epoxides as well. By using a peracid, epoxidation reactions can take place to obtain an epoxide. Then you can react with the epoxide by adding a nucleophile.
These are the important details in the reactivity of the ether and epoxide. The ether produces different compounds and regioselectivity depending on the reaction conditions, so make sure you understand these.