In organic chemistry, synthetic reactions are often carried out by acids and bases. The reaction of a Lewis acid and a Lewis base creates new bonds.
So when a compound reacts chemically, what kind of atoms bond with each other? In this regard, the HSAB theory (or HSAB principle) helps us to predict where compounds will react chemically. In the HSAB principle, acids and bases are described as hard acids and soft bases, and so on.
You don’t have to remember exactly which atoms are hard and which ones are soft because you can check the table. However, it is important to understand the concept of the HSAB theory to understand how a synthetic reaction proceeds.
Although the HSAB theory is an important rule of thumb in inorganic chemistry, organic chemistry uses the HSAB theory more frequently in laboratory experiments. So we will explain how to think about and use the HSAB theory.
Table of Contents
Judging Atoms by Their Hardness and Softness
There are several definitions of acids and bases. For these acids and bases, the Brønsted-Lowry theory is easy to understand. A molecule that gives an H+ (hydrogen atom) is a Brønsted acid, and a molecule that receives an H+ (hydrogen atom) is a Brønsted base.
However, we don’t just consider the definition of Brønsted-Lowry in organic chemistry. This is because, in the reaction between acids and bases, the H+ (proton) does not necessarily move.
So, we think of using Lewis acids and Lewis bases. When a Lewis acid reacts with a Lewis base, the HSAB principle is a method for predicting the ease of reaction.
-The HSAB Theory Is a Rule of Thumb for Making Bonds Between Acids and Bases with the Same Properties
Why is it important to learn the HSAB principle? As mentioned above, the HSAB theory is the principle that can predict how chemical reactions will occur.
The HSAB theory is as follows.
- Hard acids make strong bonds with hard bases.
- Soft acids make strong bonds with soft bases.
Making strong bonds means that the reaction is more likely to proceed. For example, if you add a hard acid, the reaction will easily proceed with a hard base instead of a soft base. This feature is the HSAB theory, and it is responsible for the ease of reaction between molecules.
The Concept of Hard and Soft Atoms (Ions)
What does it mean for an atom to be hard or soft? There are several factors that contribute to this, especially the ease of polarization is important.
Hard acids and bases are less likely to be polarized. On the other hand, soft acids and soft bases are more easily polarized.
When does a molecule become easily polarized? This is affected by the atomic radius. If the atomic radius is small, electrons are strongly attracted to the nucleus. Because the nucleus contains positively charged protons, negatively charged electrons are attracted to the nucleus and, as a result, are less likely to be polarized.
On the other hand, if the radius of an atom is large, the electrons are away from the nucleus. Therefore, they are less affected by the nucleus and are more easily polarized.
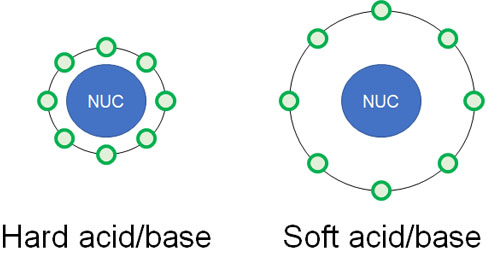
When compounds with positive or negative charges are nearby, the soft atoms are more likely to be polarized. There is this difference between hard and soft atoms.
How Do We Remember the HSAB Principle?
According to the HSAB principle, hard and soft ions can be classified as follows.
-Classification of Acids
| Hard acids | H+, Li+, Na+, Mg2+, Ca2+, Al3+, Fe3+, Si4+, BF3, AlCl3, etc. |
| Intermediate acids | Fe2+, Cu2+, Zn2+, R3C+, etc. |
| Soft acids | Cu+, Ag+, Au+, Hg+, Hg2+, BH3, RS+, I2, Br2, etc. |
-Classification of Bases
| Hard bases | H2O, OH–, F–, Cl–, NO3–, ROH, RO–, NH3, RNH2, etc. |
| Intermediate bases | Br–, N3–, NO2–, aniline, pyridine, etc. |
| Soft bases | H–, CN–, R2S, RSH, RS–, I–, R–, R3P, CO, benzene, etc. |
It doesn’t make sense to remember all of these lists exactly. You just need to learn how to remember them roughly and be able to distinguish them.
If the atomic radius is small and the negative charge is low, it is more likely to be a hard acid or base. On the other hand, if the atomic radius is large and the negative charge is high, it tends to be a soft acid or base. In addition, the HSAB theory has the following tendency.
-Atoms (Ions) Are Softer on the Lower Part of the Periodic Table
Atoms on top of the periodic table have a smaller radius. In contrast, the lower an atom is on the periodic table, the larger its atomic radius is, and the softer the acid or base.
For example, H+, alkali metals, and alkaline earth metals at the top of the periodic table are hard acids. As for bases, F– and Cl– are also hard bases. But Br– is an intermediate base, and I– is a soft base. The ionic radius plays a major role in this.
-Binding to a Hard Atom Makes the Ion Harder, and Binding to a Carbon Makes it Softer
Atoms with high electronegativity (F, O, N, etc.) are hard atoms. When bonded to these hard atoms, ions tend to be hard, such as NO3– and NH3–. On the other hand, if a molecule is bonded to carbon, it tends to become a softer ion.
Of course, it is often difficult to distinguish between these criteria alone; the HSAB theory is just a rule of thumb.
-High Valence Metal Ions Are Hard
Metal ions with high valence tend to be hard, such as Al3+, Fe3+, and Si4+.
On the other hand, Fe2+ is an intermediate acid, even for the same iron ion. If the valence changes, the hardness and softness of the ion will be different.
Reactivity Differs Between Electrostatic and Orbital Interactions
Now we understand that there are differences between Lewis acids and Lewis bases, with each molecule being harder or softer. So why do these differences occur? And why do hard ions tend to form bonds with each other and soft ones with each other?
Two factors are involved: electrostatic and orbital interactions.
The force of attraction between positive and negative charges is called electrostatic interaction. In the case of hard acids and bases with small atomic radius, electrostatic interactions play a major role in chemical reactions. Hard acids and hard bases react because they are more likely to undergo chemical reactions with acids and bases.
On the other hand, in the case of a large atomic radius, synthetic reactions often proceed due to the overlap of molecular orbitals rather than chemical reactions depending on the degree of polarization. Molecular orbitals include HOMO (binding orbitals) and LUMO (anti-bonding orbitals), and electrons move through the orbitals in the molecule due to the influence of each other.
With a large atomic radius, it is more important for the molecular orbitals to overlap to form bonds than the effects of positive and negative charges. This is why softer acids and bases are more likely to react.
The HSAB Theory Is Useful for Predicting Organic Chemistry Reactions
What is the benefit of learning HSAB theory? As mentioned above, one of the areas of inorganic chemistry is the HSAB theory. We learn the HSAB theory in complex chemistry.
However, in practice, the HSAB theory is used more often in organic chemistry than in inorganic chemistry. This is because we can predict the reactions that will occur depending on whether the reagents to be reacted are hard or soft. Therefore, we should try to distinguish between hard and soft for molecules.
For example, consider a situation when the following nucleophilic substitution reaction (SN2 reaction) occurs.
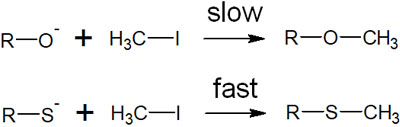
Comparing alkoxide (RO–) and thiolate (RS–), alkoxide is more basic than thiolate. Therefore, it would seem that alkoxide and iodomethane would react more quickly. In reality, however, thiolate and iodomethane react faster.
Why is this the case? This can be explained by the HSAB principle. Sulfur atoms have a larger atomic radius than oxygen atoms. Iodine ions also have a large atomic radius. This is why soft acids and bases interact with orbitals and quickly react chemically to form new bonds.
Example of Reaction with Grignard Reagent Under HSAB Principle
Learning the HSAB principle also helps us to understand regioselectivity. In organic chemistry, it is very important to predict where a reagent will react.
Compounds with magnesium metal in the molecule are Grignard reagents. Grignard reagents containing magnesium are hard nucleophiles and are strong bases. Grignard reagents are negatively charged and attack the carbonyl carbons that are positively polarized.
In other words, a chemical reaction occurs when a hard acid reacts with a hard base. The reaction is caused by acids and bases. We can explain the 1,2 addition in Grignard reagents by the HSAB theory.
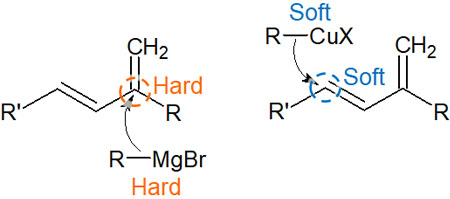
On the other hand, among organometallic compounds, the reactivity of organocopper reagents changes. Organocopper reagents are called Gilman reagents. The Gilman reagent contains copper in the molecule, and copper is a soft metal with a large atomic diameter.
Therefore, it attacks the carbon in the double bond, which is the soft part of the molecule. It attacks the double bond, not the carbonyl carbon because the effect of the overlapping molecular orbitals is stronger than the effect of the acid and base. This results in 1,4 addition (Michael addition).
The difference between the 1,2 and 1,4 addition (Michael addition) in organometallic compounds is frequently used as an example of a reaction in the HSAB theory. Of course, for other organic reactions, the HSAB theory can also be used to predict the progress of the reaction.
Predicting Synthetic Reactions Using the HSAB Theory
You can remember how chemical reactions occur. However, it’s hard to remember all. So, try to understand the rules of how a chemical reaction occurs. Then you will be able to guess the reaction mechanism without having to remember.
One of these rules is the HSAB principle. There are different types of atoms and ions, such as hard and soft. For acids and bases, they have different properties.
We don’t have to remember all the hard and soft molecules for acids and bases. Just try to learn what properties make a compound hard (or soft).
This will allow you to predict the rate and regioselectivity of the chemical reaction. The HSAB theory is used more frequently in organic chemistry than complexes in inorganic chemistry. Use the HSAB principle in organic chemistry reactions to be able to predict what kind of reaction will occur.