There are so many compounds with benzene rings. Benzene rings are called aromatics, and compounds with a benzene ring are aromatic compounds.
A molecule with double bonds is a benzene ring. However, a benzene ring undergoes an entirely different chemical reaction than an alkene. Benzene rings are electron-rich like alkenes, but they do not undergo electrophilic addition reactions.
Instead of an addition reaction, a substitution reaction occurs at the benzene ring. The chemical reaction proceeds without loss of aromaticity. This is called electrophilic aromatic substitution.
The Friedel-Crafts reaction is the most famous substitution reaction at the benzene ring. We will explain how electrophilic aromatic substitution proceeds, including reaction mechanisms.
Table of Contents
The Benzene Ring Is Stable and No Addition Reaction to the Double Bond Occurs
Benzene rings are known to be very stable molecules. In the case of an alkyl chain, an addition reaction to an alkene occurs, and the double bond becomes a single bond. On the other hand, although the benzene ring contains double bonds, addition reactions to the benzene ring do not occur.
For example, an alkane can be synthesized from an alkene by the addition reaction to a double bond as follows.
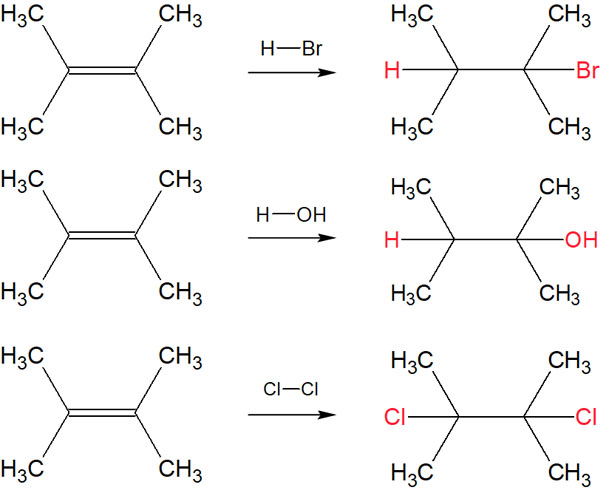
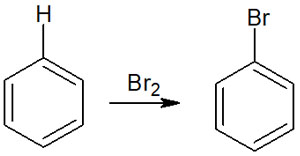
In contrast, the addition of hydrogen bromide (HBr) or bromine (Br2) to the benzene ring, for example, does not give the following compounds.
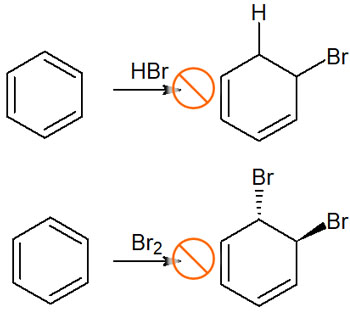
The aromatic rings are very stable, and in the figure above, the compound after the reaction has lost its aromaticity. Since we are synthesizing an unstable compound from an extremely stable compound, the activation energy is too large, and the synthetic reaction does not proceed.
The Reaction Mechanism of the Benzene Ring Is to React Without Loss of Aromaticity
What kind of synthetic reaction does an aromatic compound undergo? In the case of the benzene ring, the synthetic reaction proceeds without loss of aromaticity. In other words, even if a substituent is attached to the benzene ring and the aromaticity is momentarily lost, the next reaction takes place so that the aromaticity is restored.
In short, in an electrophilic aromatic substitution reaction, the hydrogen atom bonded to the benzene ring is replaced by another substituent. For example, it is as follows.
It is called electrophilic aromatic substitution reaction because the hydrogen atom attached to the benzene ring is replaced by another substituent.
So what is the reaction mechanism of electrophilic aromatic substitution reactions? In all electrophilic aromatic substitution, the synthesis proceeds that aromaticity is restored as described above. The reaction mechanism is as follows.
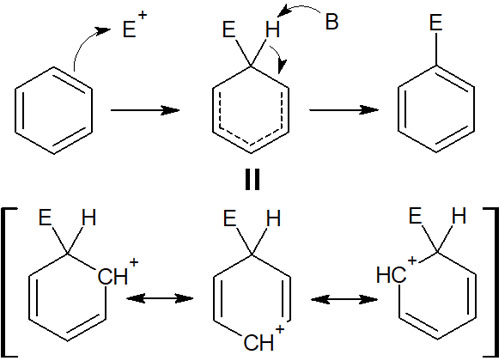
Intermediates that undergo electrophilic aromatic substitution lose their aromaticity. However, the intermediate is still stable, although unstable, because the resonance structure can be written. Subsequently, a hydrogen atom (proton) is pulled out to restore the aromaticity, completing the substitution reaction.
All electrophilic aromatic substitutions proceed in this way. Although the functional groups that bind to the benzene ring are different, the basic reaction mechanism is the same.
Nitration and Sulfonation Are Examples of Electrophilic Aromatic Substitution Reactions
What are some examples of electrophilic aromatic substitution? Typical electrophilic aromatic substitution includes nitration and sulfonation.
As the name implies, electrophilic aromatic substitution is caused by the presence of a strong electrophilic agent, which leads to the substitution of the benzene ring. Therefore, in nitration and sulfonation, strong acids such as sulfuric acid are used in the reaction.
-Nitration to Benzene Ring
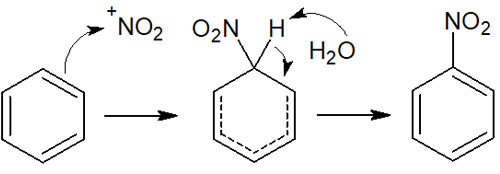
Nitrobenzene is formed when concentrated nitric acid and concentrated sulfuric acid are mixed, and benzene is added. When concentrated nitric acid and concentrated sulfuric acid are added, nitronium ions are formed. Nitronium ion is a strong electrophilic agent.
Therefore, nitration occurs by the following reaction mechanism.
-Sulfonation to Benzene Ring
Sulfonation of the benzene ring is also a typical electrophilic aromatic substitution. Sulfonation can be achieved by reacting the benzene ring with oleum (a liquid in which sulfur trioxide is absorbed into concentrated sulfuric acid).
When concentrated sulfuric acid reacts with sulfur trioxide, a strong electrophilic agent is formed. It is as follows.
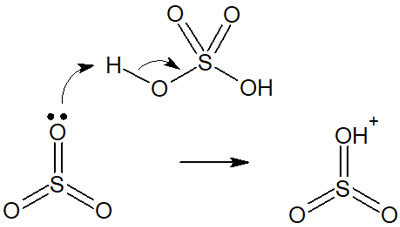
The electrophiles then react with the benzene ring as follows.
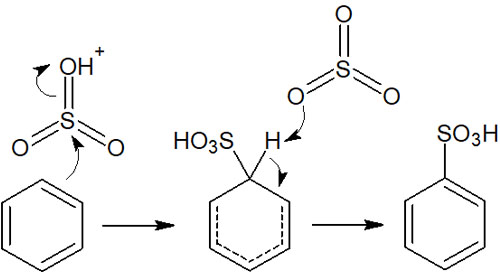
For reference, sulfonation of the benzene ring is a reversible reaction. Therefore, when it reacts with water under high-temperature conditions, the sulfo group is removed to form benzene. The synthetic reaction of benzene sulfonic acid with water is also one of the electrophilic aromatic substitution reactions.
Halogenation of the Aromatic Rings
In addition to nitration and sulfonation, there is another important reaction in electrophilic aromatic substitution, which is the Friedel-Crafts reaction. In synthetic reactions for adding substituents to benzene rings, we must study Friedel-Crafts reactions.
There are two types of Friedel-Crafts reactions.
- Friedel-Crafts Alkylation
- Friedel-Crafts Acylation
In order to understand these two reaction mechanisms, you must learn about the halogenation of the benzene ring beforehand. Once you understand the halogenation to aromatic rings, the reaction mechanism is the same for both alkylation and acylation.
As mentioned above, the benzene ring has a very stable structure, so it does not react with hydrogen chloride (HCl) or hydrogen bromide (HBr) as alkenes do. Instead, the benzene ring can be halogenated by the addition of Cl2 (chlorine) or Br2 (bromine) using Lewis acids such as FeCl3 (iron chloride) or FeBr3 (iron bromide).
In Lewis acid catalysts such as FeCl3 and FeBr3, there is an unoccupied orbital. Chlorine or bromine atoms are attached to this unoccupied orbital to produce an electrophilic agent.
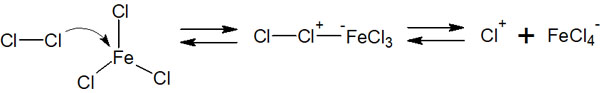
Subsequently, the benzene ring attacks the electrophiles, resulting in halogenation. The reaction mechanism is as follows.

As with nitration and sulfonation, the electrophilic aromatic substitution proceeds by an electrophilic agent. The difference is that FeCl3 and FeBr3 are used as Lewis acid catalysts instead of sulfuric acid.
Alkylation by Friedel-Crafts Reaction
An important fact is that the use of a Lewis acid catalyst allows chlorine and bromine atoms to be incorporated into the catalyst, creating a strong electrophilic agent. This property allows for alkylation to the benzene ring. This is called a Friedel-Crafts alkylation reaction.
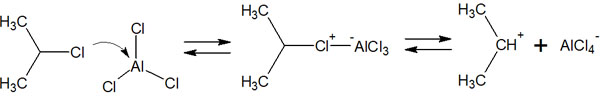
In the Friedel-Crafts alkylation reaction, AlCl3 or AlBr3 is used as a Lewis acid catalyst. By using these catalysts and alkyl halides as reagents, the benzene ring can be alkylated.
The reaction mechanism is almost the same as that of halogenation. First, a Lewis acid catalyst reacts with an alkyl halide to form a carbocation (electrophilic agent), as shown below.
The benzene ring then attacks the electrophiles to complete the alkylation.
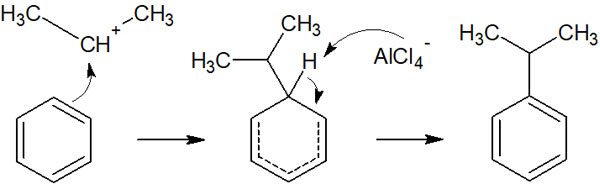
The reaction mechanism that produces an electrophilic agent is the same as for halogenation. The reaction mechanism for electrophilic aromatic substitution reactions is also the same. The difference is that the benzene ring can be alkylated using a Lewis acid catalyst.
Rearrangement Reactions in Friedel-Crafts Alkylation
There is a note of caution in the Friedel-Crafts alkylation reaction, which is a rearrangement reaction. Since this reaction involves a carbocation as an intermediate, a rearrangement reaction may occur.
The intermediates of the carbocation have an order of stability. The order is as follows.
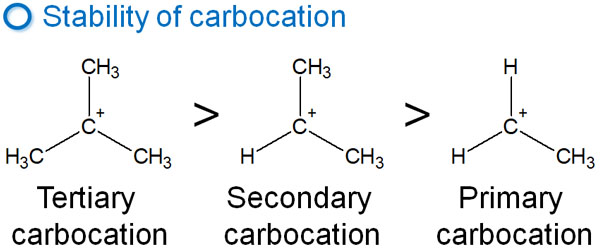
Therefore, after the carbocation is formed, a more stable carbocation is formed by transferring the hydrogen atom to the neighboring carbon. For example, it looks like the following.
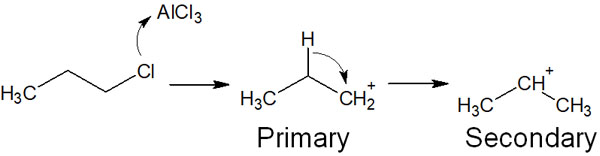
By reacting with a Lewis acid catalyst, the primary carbocation is initially formed. However, the intermediate is more stable than this state if the hydrogen atom is transferred to a neighboring carbon atom to form a secondary carbocation. Therefore, the hydrogen atom is transferred to become a more stable carbocation.
Because of the carbocation rearrangement, you may get a product that is different from the expected compound. This is due to the carbocation rearrangement.
-Formation of Polysubstituted Compounds Is Likely to Be a Problem
It should be noted that in Friedel-Crafts alkylation reactions, polysubstituted benzene rings can be easily synthesized.
Carbon is known to push out electrons. Therefore, when an alkyl chain is attached to a benzene ring, the electron density of the aromatic ring increases. As a result, the increased reactivity of the benzene ring frequently results in polysubstituted compounds as well as compounds with a single substituent attached. It is as follows.
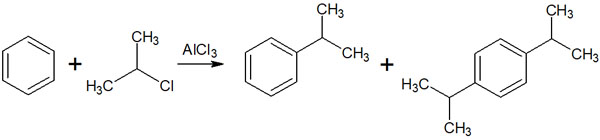
The alkyl chain of the benzene ring is ortho-para oriented. This leads to the formation of compounds with alkyl chain substituents in the ortho and para positions.
This situation can be avoided by increasing the amount of benzene. When benzene is present in excess, the reagent has a higher probability of reacting with benzene than the compound produced by alkylation. As a result, the synthesis of polysubstituted benzene is avoided.
Acylation to Benzene Rings: Friedel-Crafts Acylation
In addition to alkylation, the Friedel-Crafts reaction is also used for acylation. This is called the Friedel-Crafts acylation reaction.
In the Friedel-Crafts acylation, acyl halides are used as reagents. The addition of a Lewis acid, AlCl3, allows the Friedel-Crafts reaction to proceed. The reaction mechanism for the formation of an electrophilic agent is as follows.
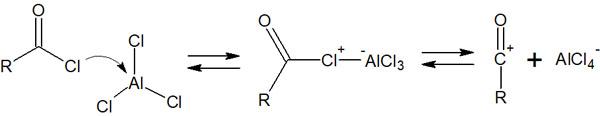
An acyl cation is generated as an electrophilic agent. Subsequently, the electrophilic aromatic substitution proceeds as follows.
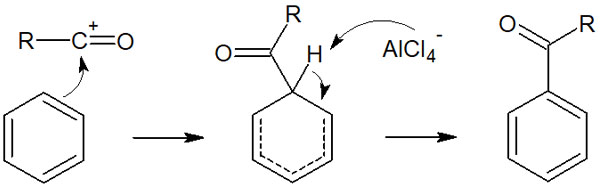
For Friedel-Crafts acylation reactions, the reaction mechanism is simple if you understand what we have discussed so far. The reaction mechanism is the same, and the only difference is the use of an acyl chloride compound as a reagent.
-Friedel-Crafts Acylation with Acid Anhydride
In the Friedel-Crafts acylation reaction, not only acyl halides but also acid anhydrides undergo Friedel-Crafts acylation.
Acyl chloride reacts with aluminum chloride (AlCl3) to produce acyl cations. In other words, when the acyl cation, which is an electrophilic agent, is formed, the Friedel-Crafts acylation proceeds. When the acid anhydride reacts with AlCl3, an acyl cation is produced, as shown below.
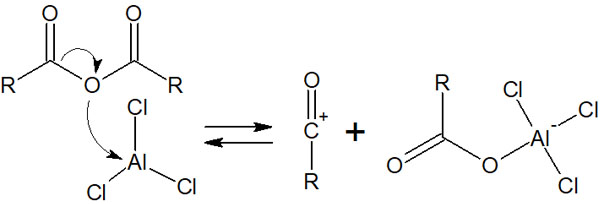
In this way, an acyl cation is created, and the Friedel-Crafts acylation reaction described earlier occurs. Since the reaction mechanism is the same, we omit the explanation.
-There Will Be No Two Acyl Groups Bonded
Earlier, we explained that Friedel-Crafts alkylation reactions produce polysubstituted compounds. On the other hand, in Friedel-Crafts acylation, there will not be two acyl groups attached to the benzene ring.
When an acyl group is attached to an aromatic ring, the acyl group becomes an electrophilic functional group. This reduces the electron density of the benzene ring and makes the aromatic ring less reactive. This is why we do not have to worry about the formation of polysubstituted compounds in Friedel-Crafts acylation reactions.
Electrophilic Aromatic Substitution of Benzene Ring Has the Same Reaction Mechanism
One of the most important molecules is the benzene ring. Many organic compounds contain benzene rings, and it is important to understand how substituents can be added to aromatic rings.
However, aromatic rings are different in nature from the double bonds of alkenes. In aromatic rings, addition reactions do not occur, but electrophilic substitution reactions occur. The aromaticity is not lost, and after the chemical reaction, the benzene ring is restored to give the product.
The reaction mechanism is the same for all electrophilic aromatic substitutions. Therefore, the reaction mechanism is easy to understand, although there are different types of reactions, such as nitration, sulfonation, halogenation, Friedel-Crafts alkylation, and Friedel-Crafts acylation.
Just be careful about rearrangement reactions, orientation, and the formation of polysubstituted compounds. In this way, substituents can be put into the benzene ring.