It is important to understand how the different functional groups react with each other in the synthesis. Among these functional groups, ketones and aldehydes are known to be two of the most important functional groups.
Ketones are called carbonyl groups, and aldehydes are called formyl groups. What they have in common is that they both have a C=O structure. Therefore, in organic chemistry, both carbonyl and formyl groups undergo similar chemical reactions.
Carbonyl compounds, including ketones and aldehydes, cause various types of chemical reactions. They are very important functional groups in organic chemistry because we can synthesize many kinds of compounds.
However, there are many types of reactions, and their reactions tend to be complicated. In this section, we will explain the nucleophilic addition to carbonyl compounds, which is one of the most common chemical reactions of carbonyl compounds.
Table of Contents
Carbon Atoms in Carbonyl Compounds Are Electron Deficient
In organic chemistry, we deal with a variety of functional groups. Among these functional groups, carbonyl compounds are characterized by one thing. The carbons in carbonyl compounds are electron-deficient.
In the case of carbonyl and formyl groups, there is an oxygen atom attached to the carbon atom. And they’re not connected by a single bond but by a double bond. Therefore, electrons in the double bond can be transferred to the oxygen atom.
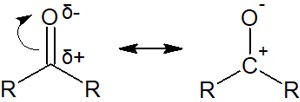
The carbon atom is positively charged and is connected to the oxygen atom by a double bond, making it easier for the nucleophile to attack the carbon atom. This is a fact that we must recognize in synthetic reactions using carbonyl compounds.
In the Absence of Leaving Groups, Nucleophilic Addition Reaction Occurs
In the case of carbonyl and formyl groups, there are no leaving groups. In the case of ketones, there are alkyl chains next to the C=O carbon atom. For aldehydes, the alkyl chain and the hydrogen atom are next to the C=O carbon atom. Therefore, nucleophilic addition reactions occur for carbonyl and formyl groups.
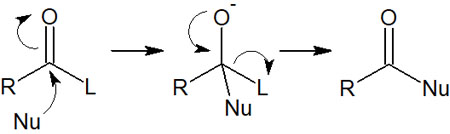
The reaction proceeds when a nucleophile (Nu) attacks the carbonyl carbon, as shown below.
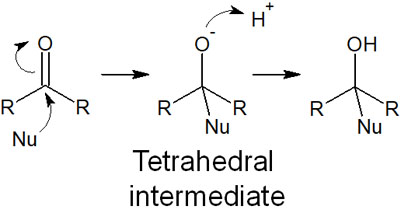
After the nucleophilic agent attacks the carbonyl carbon, the carbon atoms form sp3 hybrid orbitals (tetrahedra). The intermediates formed in this process are called tetrahedral intermediates. Whenever a nucleophile attacks a carbonyl carbon, a tetrahedral intermediate is produced.
However, the state of the oxygen atom with a negative charge is unstable. Therefore, the oxygen atom attacks the hydrogen atom (proton) to form -OH. In this way, the synthetic reaction is completed.
If There Is a Leaving Group, a Nucleophilic Acyl Substitution Reaction Occurs
There are many types of carbonyl compounds. Although carbonyl and formyl groups do not have a leaving group, there are other carbonyl compounds with a C=O structure, such as the following compounds.
- Carboxylic acid
- Ester
- Amide
- Acyl halide
- Acid anhydride
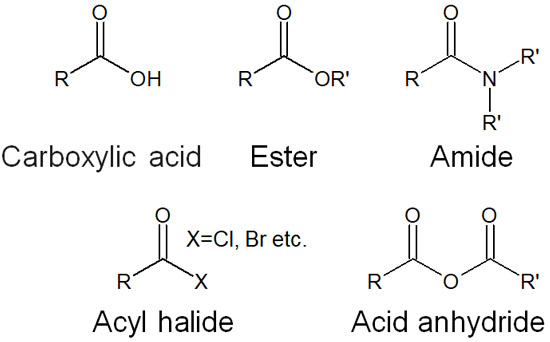
These compounds are called carboxylic acid derivatives. They are not bonded with alkyl chains like carbonyl groups. Instead, highly electronegative atoms such as oxygen and nitrogen atoms are bonded next to the carbon atom.
In the case of these carboxylic acid derivatives, substitution reactions occur because of the presence of highly electronegative atoms next to the carbon atom. The following nucleophilic acyl substitution reactions occur.
Nucleophilic addition reactions, which occur with carbonyl and formyl groups, do not occur. The type of chemical reaction that occurs depends on the presence of the leaving group.
Nucleophilic Addition Reaction with Carbonyl Compounds
If we explain the reactions of carbonyl compounds, including carboxylic acid derivatives, the contents would be very extensive. Therefore, we will discuss the nucleophilic addition reactions of carbonyl and formyl groups, which have no leaving group.
There are several types of nucleophilic addition reactions to ketones and aldehydes. Among them, the most important synthetic reactions are as follows.
- Grignard reaction
- Synthesis of cyanohydrin
- Synthesis of Amines: Imine and Enamine
- Hydration reaction: acetal and hemiacetal
The reagents used are different, and the compounds produced are different. However, the reaction mechanisms are all nucleophilic additions to carbonyl compounds. The following is an explanation of how the synthetic reactions are carried out for ketones and aldehydes.
Synthetic Reactions with Grignard Reagents
Organometallic compounds are very strong bases. Organometallic compounds react with carbonyl and formyl groups to form hydroxy groups (-OH).
There are different types of organometallic compounds, and the most famous compound is the Grignard reagent. Compounds with magnesium (Mg) in the molecule are Grignard reagents, and the Grignard reaction results in a nucleophilic addition reaction. A compound with the following structure is a Grignard reagent.
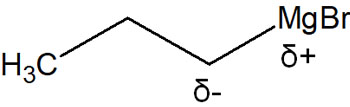
In the Grignard reagent, the carbon atom bonded to magnesium has a negative charge. Therefore, the synthesis proceeds in the same way as carbanions (negatively charged carbon atoms).
What is the reaction mechanism of the Grignard reaction? In this regard, the anionic carbon of the Grignard reagent attacks the carbonyl carbon. At the same time, the oxygen atom and the magnesium bond. The result is as follows.
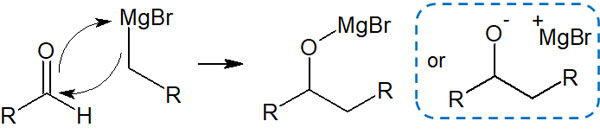
Tetrahedral intermediates, in which the oxygen atoms are negatively charged, are unstable. However, since organometallic compounds are extremely basic, they can exist as tetrahedral intermediates.
Subsequent treatment, such as the addition of water, will cause the magnesium atom to disappear from the oxygen atom, and a hydrogen atom is bonded to the oxygen atom. As a result, alcohol is synthesized.
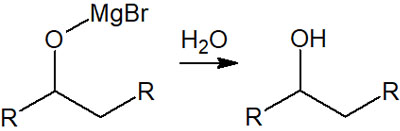
Grignard reactions to ketones and aldehydes can be used to make new carbon chains. Therefore, it is one of the most important synthetic reactions in organic chemistry.
Synthesis of Cyanohydrin in the Presence of Proton
Even if the reagent is not a strong base such as an organometallic compound, the carbonyl carbon is subject to nucleophilic attack. Cyanide ions, for example, are known to be nucleophiles.
In the synthetic reaction of cyanohydrin, HCN (hydrogen cyanide) is used. After the cyanide ion attacks the carbonyl carbons, the oxygen atoms capture the hydrogen atoms (protons) present in the solution. The result is the formation of cyanohydrin.
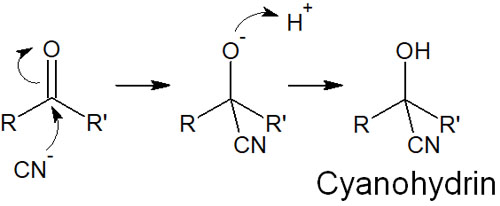
The important thing is the presence of a proton in the solution. If there is no H+ in the solution due to a strong base, the cyanide ion will become a leaving group and return to its original state.
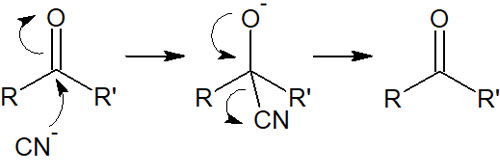
Since the cyano groups have the ability to leave, cyanohydrin can be synthesized only when there are protons in the solution.
Reactions with Amines to Form Imines or Enamines
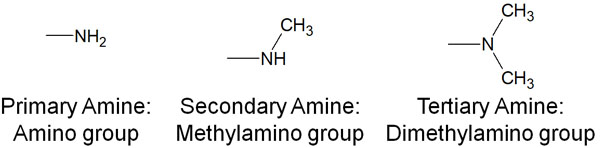
Amines are compounds that react with ketones and aldehydes through a reaction mechanism similar to that of the cyanide ion described earlier. Amines are classified into primary amines, secondary amines, and tertiary amines according to the number of carbon chains attached to them, as shown below.
For these amines, when H+ is present in the solution, the following reaction proceeds in the same way as for cyanide ions.
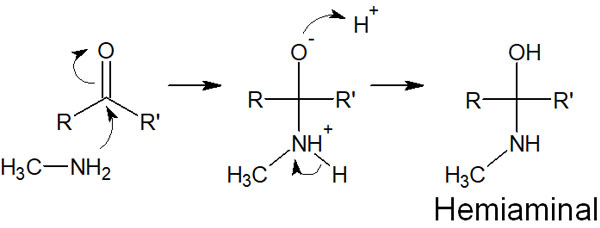
The compound that is formed is called hemiaminal. The difference from the cyanohydrin synthesis just described is that the nitrogen atoms are positively charged after the amine attacks. Therefore, in primary and secondary amines, the protons are removed, and the positive charge is lost.
Tertiary amines, on the other hand, have no protons to be removed. Amines with a positive charge are excellent leavening groups, so even if a tertiary amine attacks the carbonyl carbon, it will be restored, as shown below.
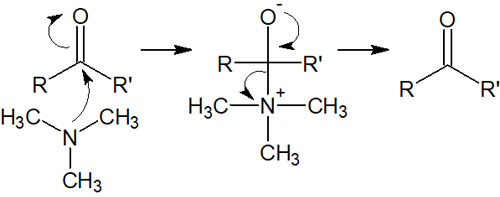
In other words, if a tertiary amine reacts with a carbonyl compound, the synthetic reaction will not proceed. When reacting with a primary amine or a secondary amine, the synthetic reaction with the carbonyl compound proceeds.
-Primary Amines React with Ketones or Aldehydes to Form Imines
Unlike the Grignard reaction and cyanohydrin synthesis, the next reaction proceeds. This is because the product, hemiaminal, is an unstable substance.
When a primary amine reacts with a ketone or aldehyde, an imine is formed from the hemiaminal. The reaction mechanism is as follows.
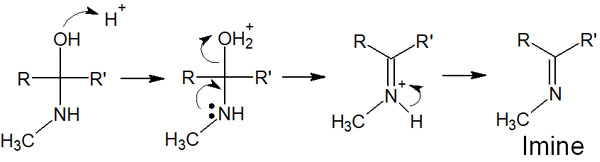
In the formation of imine, imine is made by the formation of a double bond between a nitrogen atom and a carbon atom. Due to the presence of a hydrogen atom in the nitrogen atom, the double bond is formed as soon as the H+ disappears.
-Secondary Amines React with Ketone or Aldehyde to Form Enamine
On the other hand, what happens when a secondary amine reacts with a ketone or aldehyde? In the case of a secondary amine, no hydrogen atoms are bonded to the nitrogen atom, even if it forms a hemiaminal. Therefore, a double bond cannot be formed between the nitrogen atom and the carbon atom and thus cannot become an imine.
Instead, the reaction of a secondary amine with a carbonyl compound yields an enamine. The reaction mechanism is as follows.
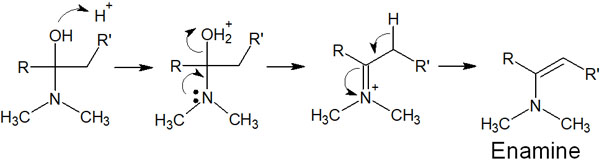
Since there is no hydrogen atom bonded to the nitrogen atom, it is impossible to create a double bond between nitrogen and carbon. Instead, the hydrogen atom (proton) bonded to the carbon atom is pulled out to produce an alkene. A compound with an N-C=C structure is an enamine.
It is not difficult to understand the reaction mechanism of amines and carbonyl compounds to produce hemiaminals. However, with amines, the synthetic reaction continues to proceed afterward. Moreover, the product depends on whether it is a primary or secondary amine.
- Reaction with primary amines: imines are formed.
- Reaction with secondary amines: enamine is formed.
The reaction mechanism is complicated, and try to understand what kind of compound you will get.
The Hydration Reaction Produces Acetal and Hemiacetal
For compounds with carbonyl and formyl groups, there are other important synthetic reactions. These are acetal and hemiacetal. These are called hydration reactions.
In the presence of an acid catalyst, a proton binds to the carbonyl oxygen, causing it to have a positive charge. The alcohol then attacks the carbonyl carbon, producing a hemiacetal. The reaction mechanism is as follows.
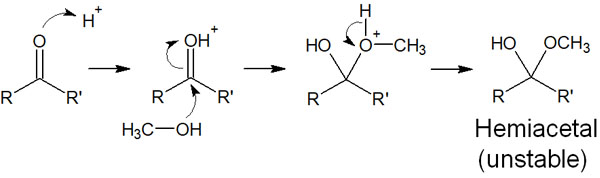
The reaction mechanism of hemiacetal is almost the same as the reaction mechanism we have explained so far. Therefore, you can understand it without any problem.
-Acetal Is Obtained from Hemiacetal
However, hemiacetal is unstable. We explained earlier that the hemiaminals produced in amine synthesis are unstable, so imines or enamines are produced. The same is true for the hydration reaction of carbonyl compounds, and further reactions are required to obtain the products.
Since the synthetic reaction is carried out under an acid catalyst, the hydroxy group (-OH) is able to capture a proton. As a result, oxonium ion is produced by the removal of H2O. However, the oxonium ion is an unstable intermediate, and the alcohol will nucleophilically attack it. As a result, acetal is synthesized.
The mechanism of this reaction is as follows.
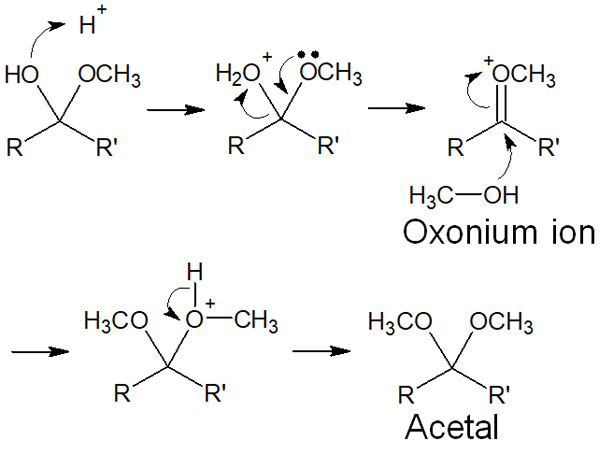
In other words, understand that the hydroxy group (-OH) is replaced by an alkoxy group (-OR).
Why is the synthesis of acetal important? Because it is a protecting group for the functional group. The synthesis of acetal is a reversible reaction. This means that you can convert the acetal back to a ketone or aldehyde.
As we’ve discussed, carbonyl carbons are highly reactive. Therefore, the addition of strongly basic reagents such as amines leads to nucleophilic addition reactions. On the other hand, if you convert it to acetal beforehand, the carbonyl group is not present and not be attacked by nucleophiles.
It can then be converted back to a compound with a carbonyl or formyl group by hydrolysis when needed.
There Are Many Types of Synthesis for Carbonyl Compounds
We have discussed the synthetic reactions of carbonyl compounds that do not have a leaving group. In carboxylic acid derivatives, nucleophilic acyl substitution reactions occur. On the other hand, nucleophilic addition reactions occur in carbonyl compounds without any leaving groups.
Once again, the following synthetic reactions are important in nucleophilic addition reactions.
- Grignard reaction
- Synthesis of cyanohydrin
- Amine reactions: synthesis of imine or enamine
- Hydration reaction: Synthesis of acetal and hemiacetal
The reaction mechanism is the same for all of them. However, for amine reactions and hydration reactions, the compounds obtained after synthesis are unstable. Therefore, it is important to understand that the synthetic reaction proceeds further. In short, amine reactions and hydration reactions have more complex reaction mechanisms.
Carbonyl compounds are an important functional group because of the many types of reactions. Since many substituents can be synthesized from carbonyl and formyl groups, you need to understand what kind of chemical reactions take place.