When synthesizing a compound with a six-membered ring, there is one synthetic reaction to consider first; the pericyclic reaction. Among the pericyclic reactions, the most famous is the Diels-Alder reaction.
In organic chemical reactions, Lewis acids and Lewis bases are usually involved in the reaction. In the pericyclic reaction, however, acids and bases are not involved in the synthetic reaction. There are no anionic or cationic intermediates, and the cyclic compounds are synthesized by the electrons moving at once.
The reaction mechanism of the pericyclic reaction is complex because the shape of the compound changes significantly. It is also necessary to take stereochemistry into account to predict the products.
For this reason, pericyclic reactions tend to be complicated. We will discuss the Diels-Alder reaction, which is the most important of all pericyclic reactions.
Table of Contents
The Most Famous Pericyclic Reaction Is the Diels-Alder Reaction
A typical pericyclic reaction is the Diels-Alder reaction. When learning about pericyclic reactions, everyone must study the Diels-Alder reaction first.
For example, the following reaction is a Diels-Alder reaction.
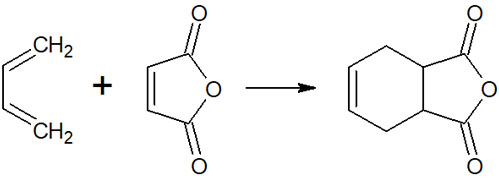
The reaction shown in the above figure proceeds simply by heating the compounds. No Lewis acids and bases are present, and no catalyst needs to be added. A six-membered ring compound can be synthesized without any intermediates.
The Diels-Alder reaction was discovered by the German chemists Otto Diels and Kurt Alder. They were awarded the Nobel Prize in Chemistry in 1950 for this discovery.
Reaction Mechanism of Conjugated Dienes and Dienophiles (Alkenes)
What is the reaction mechanism of the Diels-Alder reaction? How is a cyclic compound synthesized?
The Diels-Alder reaction always requires two compounds. One is a conjugated diene, a compound in which two double bonds are conjugated. The other compound we use is an alkene. Not just any alkene can be used, but a molecule called dienophile is used.
In the previous synthetic reaction, the conjugated diene and the dienophile are respectively as follows.
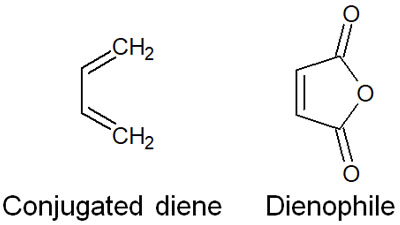
The electrons of the two compounds move to form a six-membered ring transition state, resulting in the synthesis of a six-membered compound. The reaction mechanism is as follows.
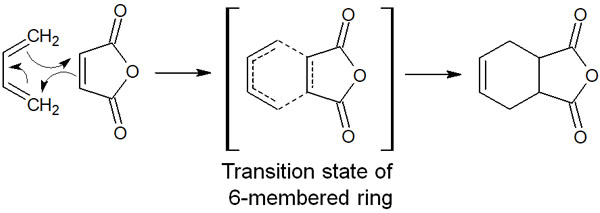
One of the reasons for the Diels-Alder reaction to proceed is the delocalization of electrons in the transition state. In the transition state, the delocalization of electrons occurs in the state of the six-membered ring. Since electrons are present everywhere in the six-membered ring, it is a stable state, just like an aromatic ring.
Note that the Diels-Alder reaction is called cycloaddition since a cyclic compound can be obtained by the addition of two compounds. There are three main types of pericyclic reactions. One of them is cycloaddition.
Reacts with Electron-Rich Conjugated Dienes and Alkenes with Electron-Withdrawing Groups (Dienophiles)
If you mix a conjugated diene and an alkene and heat them, will the Diels-Alder reaction occur? Of course, the pericyclic reaction does not always proceed.
When the Diels-Alder reaction proceeds, the conjugated diene should be in an electron-rich condition.
Also, the alkene is preferred in an electron-deficient condition. To be more precise, dienophiles must have electron-withdrawing groups bound to them. For example, typical dienophiles include molecules with the following functional groups or compounds.
- Aldehyde (Formyl group)
- Ketone (carbonyl group)
- Carboxylic acid
- Ester
- Nitrile (cyano group)
- Maleic anhydride
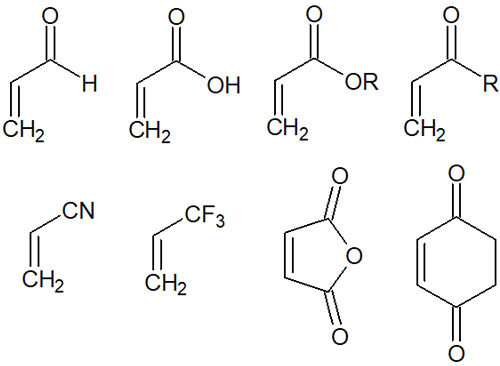
Thus, when an electron-withdrawing group is attached to an alkene, the Diels-Alder reaction is more likely to occur.
In addition to carbonyl and formyl groups, there are many other electron-withdrawing groups, such as nitro groups. When these electron-withdrawing substituents are present, they are ideal as dienophiles.
Cycloaddition Occurs in the s-cis Form, Not in the s-trans Form
Conjugated dienes, on the other hand, include cyclopentadiene, furan, and cyclohexa-1,3-diene. The reaction of these conjugated dienes with alkenes leads to the Diels-Alder reaction.
However, there are two types of conjugated dienes: s-cis and s-trans. They are as follows.
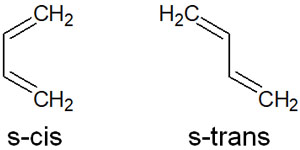
Of these, only the s-cis form is involved in the Diels-Alder reaction. Even if it is a conjugated diene, an s-trans compound cannot be involved in the Diels-Alder reaction because it cannot enter the transition state of a six-membered ring.
Therefore, when a double bond is connected by a single bond, it must be in the s-cis form.
In addition, some molecules have a fixed s-cis or s-trans form. For example, the following compounds fall into this category.
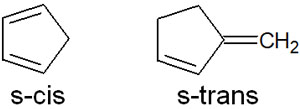
In this case, the s-trans compound cannot undergo a Diels-Alder reaction. If the conjugated diene is not in the s-cis form, no cycloaddition will occur.
Stereochemistry of the Diels-Alder Reaction: Stereoselectivity
In organic chemistry, pericyclic reactions are complex chemical reactions. Why do pericyclic reactions tend to be complicated? It is because it is necessary to consider stereochemistry.
Molecules are three-dimensional, not flat. Therefore, we need to understand the stereoselectivity and what the conformation of the compound is after synthesis.
What is important is the fact that in the Diels-Alder reaction, the synthesis proceeds while maintaining the three-dimensional structure. As mentioned above, the Diels-Alder reaction has no intermediate; after the transition state of the six-membered ring, electrons are transferred at once to cause cycloaddition. This is the reason why the synthesis proceeds with maintaining the 3D structure.
To be more specific, the following compounds can be synthesized
- A dienophile is a cis form: the product is cis.
- A dienophile is a trans form: product is trans.
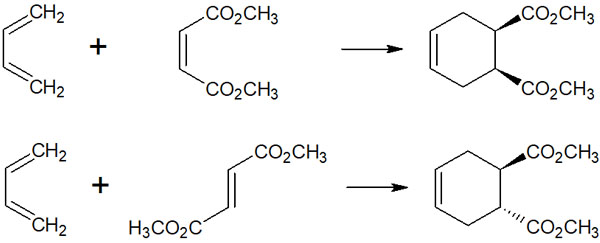
If the dienophile is cis, then the cis is retained. In other words, the product is cis form. On the other hand, if the dienophile is trans, then the product is trans form.
As the Diels-Alder reaction proceeds, the conjugated diene attacks from the top of the dienophile. For example, the following shows a conjugated diene attacking a trans dienophile.
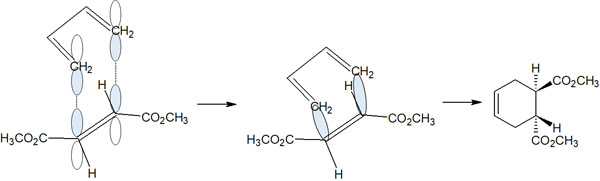
Since the reaction proceeds while the 3D structure is retained, the ester and the hydrogen atom are in the same location in the product obtained after the reaction. Therefore, we get a trans product from a trans dienophile.
Understanding the Stereochemistry of Conjugated Dienes with Alkynes (Triple Bonds)
On the other hand, how should we consider the stereochemistry of conjugated dienes? In the case of conjugated dienes, there are two types of isomers.
- cis-cis
- cis-trans
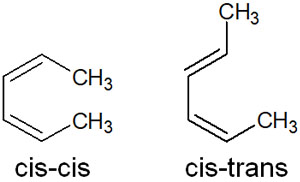
In the case of unsymmetrical conjugated dienes, trans-cis and trans-trans isomers are also added. This makes the stereochemistry more complex.
When learning about the isomers of conjugated dienes, it is easier to understand if you use alkynes (triple bonds) as dienophiles. This is because there are no isomers in alkynes. For example, when cyclopentadiene reacts with an alkyne, the reaction mechanism is as follows.
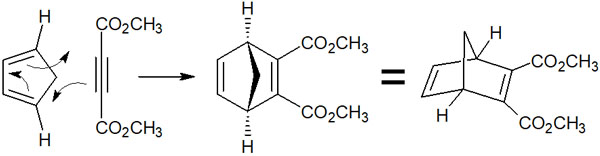
Diene has both inner and outer substituents. In the case shown above, there are two substituents. One is a methylene group, which makes a carbon bond inside the diene. The other is a hydrogen atom, which is located outside the diene.
When this molecule reacts with alkynes and causes Diels-Alder reaction, the methylene group is located at the top of the molecule, and the hydrogen atom is located at the bottom (outside) of the molecule.
In the same way, a cis-cis or cis-trans conjugated diene will react as follows.
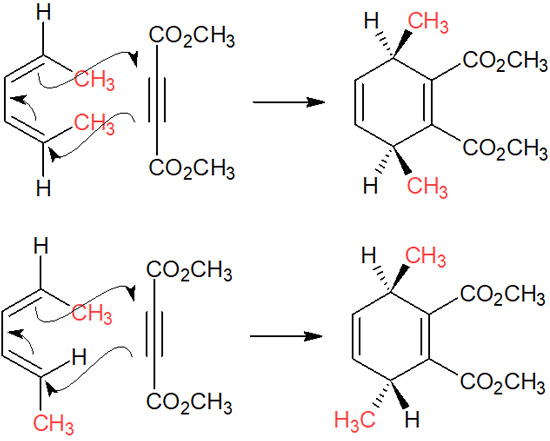
For the substituents of conjugated dienes, the inner functional groups are on the upper side of the product. On the other hand, the outer functional groups are on the lower side of the product.
In fact, cis-trans (or trans-cis) compounds are rare because the synthesis of E, Z dienes is very difficult, and there are few reports using E, Z dienes. In any case, the Diels-Alder reaction proceeds by such stereoselectivity.
Isomers of Endo-Addition and Exo-Addition
What kind of compounds can we obtain if there are isomers in both the conjugated diene and the dienophile? The reaction mechanism is more complicated because we need to take into account the stereochemistry of the two compounds.
For example, when cyclopentadiene reacts with an alkene (double bond), the following two compounds may be formed.

When the substituents of diene and dienophile are located on opposite sides of the newly formed bond by the Diels-Alder reaction, they are called exo-isomers. This addition reaction is called exo addition.
In contrast, when the substituents of diene and dienophile are on the same side of the bond, it is called endo-isomers. The addition reaction, in this case, is endo addition. The exo- and endo-isomers are as follows.
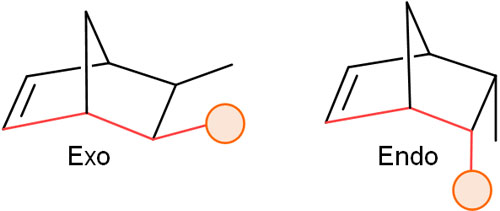
Which is synthesized, the exo- or endo-isomer? In the Diels-Alder reaction, endo-isomers are generally synthesized. This is called the endo rule.
Why the Endo Rule Takes Precedence: Secondary Orbital Interactions
Why does the endo rule take precedence? When comparing exo-isomers and endo-isomers, the exo-isomer is more stable.
In exo-isomers, the larger substituents are in the equatorial position. In the endo-isomer, on the other hand, the larger substituents are in the axial position. In cyclic compounds, it is known that the presence of a substituent in the axial position increases the steric hindrance. This is the reason why exo-isomers are more stable.
Even though exo-isomers have a more stable structure, why are endo-isomers preferentially synthesized? The reason for the endo rule has to do with secondary orbital interactions.
In dienophile, electron-withdrawing groups are bonded. C=O (carbonyl group) and C≡N (cyano group) are known as electron-withdrawing groups, which have double or triple bonds. When such functional groups with π-electrons are present in the dienophile, it tends to proceed through the end rule.
In the Diels-Alder reaction, the π orbitals of the conjugated diene and dienophile overlap. Subsequently, the π bonds disappear and σ bonds are created by the transfer of electrons. When exo addition occurs, the π orbitals of the conjugated diene and dienophile overlap at two places.
In the figure below, the orbitals of cyclopentadiene and maleic anhydride are connected at two points, resulting in exo addition.
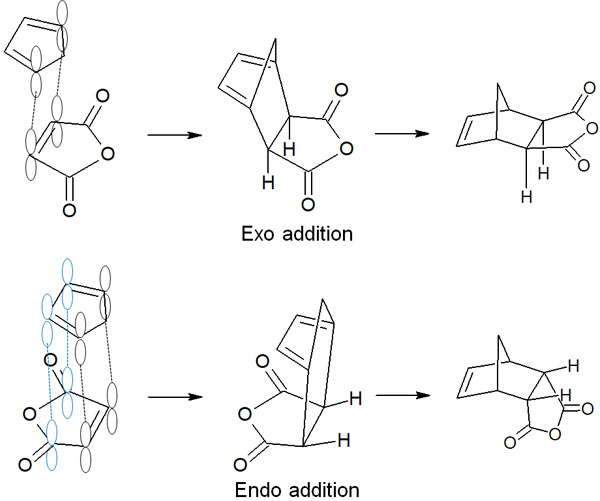
On the other hand, what about the endo addition? Conjugated dienes have two double bonds, so they have four π orbitals. In addition, when dienophiles have π bonds, such as C=O bonds, the orbitals of the conjugated diene and dienophiles overlap at four (or three) places in the endo addition. This is called secondary orbital interactions.
Due to the effects of secondary orbital interactions, endo addition takes precedence over exo addition. Although the product is less stable, the reaction takes place with less activation energy, resulting in the formation of an endo-isomer.
Even though the structure of the product is unstable, it reacts quickly with lower activation energy, resulting in the synthesis of a compound, which is called kinetic control. The endo rule of the Diels-Alder reaction is due to kinetic control, and since the reaction is irreversible, endo-isomers are synthesized.
Thermodynamic Control, Steric Hindrances, and Intramolecular Reactions Result in Exo Addition
However, it is not the case that the endo rule applies to all Diels-Alder reactions. Although the kinetic control is often caused by secondary orbital interactions, exo addition can occur predominantly in some cases.
One reason for exo addition is that the reaction is reversible. In the case of an irreversible reaction, as explained earlier, the kinetic control leads to the endo rule. However, in a reversible reaction, even though an endo-isomer is initially formed, the structure will change to a more stable exo-isomer over time.
When a stable compound is synthesized, it’s called thermodynamic control. We have just described cyclopentadiene as a conjugated diene. When furan is used instead of cyclopentadiene, the exo-isomer can be obtained through a reversible reaction.
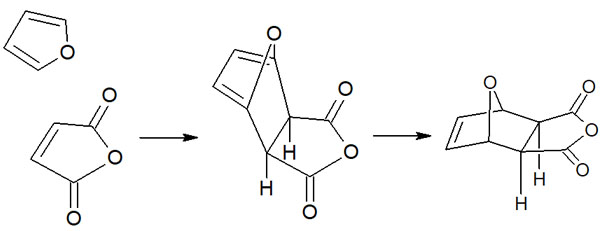
The retro Diels-Alder reaction occurs under high-temperature conditions, such as when the reaction is performed at 20°C or higher. As a result, the conformation gradually changes from endo to exo.
Alternatively, exo addition can also occur in the case of steric hindrance or intramolecular Diels-Alder reaction. If the steric hindrance of the compound to be synthesized is large, the exo-isomer is synthesized instead of following the endo-rule.
Also, in the intramolecular Diels-Alder reaction, the alkyl chain does not have the ability to move freely. Because of this limited mobility, exo addition is often preferentially caused. Although the end rule is often followed, exo-isomers can be obtained.
Consider the Diels-Alder Reaction in Three Dimensions
One of the most difficult areas of organic chemistry is the pericyclic reaction. The reason why the content can be so difficult is that you have to consider stereochemistry. Also, you have to consider how easy it is for the reaction to occur, taking into account the orbitals rather than the acid/base reaction. The shape of the molecule changes dramatically, which is another reason why it can be so complicated.
There are several types of pericyclic reactions, and a typical reaction is the Diels-Alder reaction. The reaction of conjugated dienes with dienophiles yields six-membered ring compounds.
- If the conjugated diene is in the s-cis form, then cycloaddition occurs.
- Three-dimensional is retained, and the synthetic reaction proceeds.
- Often, compounds that follow the endo rule can be obtained.
Learn the characteristics, reasons, and reaction mechanisms of these reactions. You will be able to understand the Diels-Alder reaction by thinking of molecules in three dimensions.





