A synthetic reaction to create a new carbon chain is the use of enol and enolate. The α-carbon of the carbonyl group (the carbon next to the carbonyl carbon) plays an important role, and due to its high acidity, the proton (H+) is pulled out by the base.
Therefore, the carbon next to the carbonyl group is the starting point for the formation of the enol and enolate. Then, by adding the compound you want to react with, you can alkylate it and create a new carbon chain.
For the synthesis of enolates, which is important in organic chemistry, you must understand in advance the reagents to be used and the regioselectivity of the reaction. Of course, you also need to learn about the reaction mechanism.
In the field of enols and enolates, there are several reactions that are important in organic chemistry, such as Claisen condensation, aldol reaction, and Michael addition. In order to learn these synthetic reactions, we need to understand the nature of the alpha carbon of the carbonyl group, so we will explain what the properties are.
Table of Contents
The Alpha Carbon of the Carbonyl Group Is Highly Acidic
Compounds with a carbonyl group can be used in a variety of synthetic reactions. In the case of carbonyl groups, the carbon and oxygen atoms are connected by a double bond, allowing electrons to be transferred to the oxygen atom.
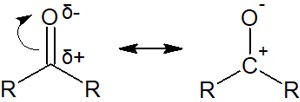
Therefore, in the presence of a nucleophile, the carbonyl carbon is attacked. This is called a nucleophilic addition reaction.
However, in carbonyl compounds, a hydrogen atom bonded to a neighboring carbon atom can be pulled out. When a proton is withdrawn by a base, the following compounds are formed.
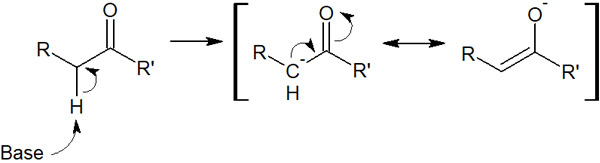
The important thing is that it gives rise to a carbanion. Because of the presence of the carbanion, it is an unstable substance. But because we can write a resonance structure, when the proton is pulled out by a strong base, we get this molecule. This molecule is called an enolate.
Because we can write a resonance structure, the hydrogen atom next to the carbonyl group is highly acidic and making it easy for a base to pull out a proton (H+).
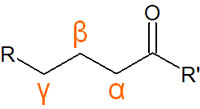
The carbon next to the carbonyl group is called the α-carbon. We distinguish the α-, β-, and γ-positions starting from the carbonyl group, as shown below.
In enolate, the alpha carbon of the carbonyl compound is an important factor. The formation of enolate ions as intermediates leads to a variety of chemical reactions.
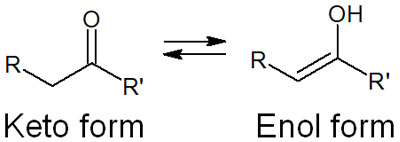
Keto-Enol Tautomerism and Enol/Enolate
In carbonyl compounds, the normal state is called the keto form. However, when a strong base is present, an enolate is created. This is called keto-enol tautomerism. The reaction mechanism is as shown earlier.
A compound with a C=O structure is the keto form. On the other hand, if the -OH is attached to an alkene, it is called the enol form. Due to keto-enol tautomerism, both keto and enol forms can change their forms.
In the enol form, if the compound has the structure of -OH, it is called an enol. On the other hand, if an oxygen atom has a negative charge, it is called an enolate. They are as follows.
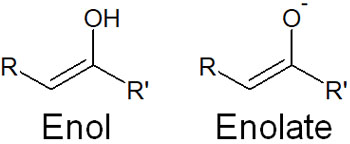
In the case of enol and enolate, it is the enolate that is more important. Enolate is produced as a result of proton withdrawal by a strong base.
Nucleophilic Enolates Make Carbon Bonds in SN2 Reactions
Why is enolate important in organic chemistry? That’s because the α-carbon of enolate is negatively charged and has nucleophilic properties as a carbanion.
As mentioned above, enolate is generated by using a strong base. The enolate then undergoes the SN2 reaction as a nucleophile. The following nucleophilic substitution reaction can be used to create a new carbon chain.
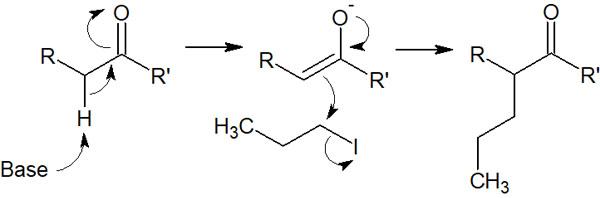
Initially, the enolate is synthesized by adding a base as a reagent. Then, the SN2 reaction proceeds by adding an alkyl halide.
Do not add an alkyl halide until after the enolate is formed by the addition of the base. The next reaction must proceed after the enolate has been synthesized. Also, the amount of base to be added is one equivalent. If the base is present in the solution, it is more likely to react with the alkyl halide.
LDA, a Bulky Base with Large Steric Hindrance, Is Important for Enolate Formation
Carbonyl groups are known to be highly reactive. By adding a base, a nucleophile attacks the carbonyl carbon, resulting in a nucleophilic addition reaction, as shown below.
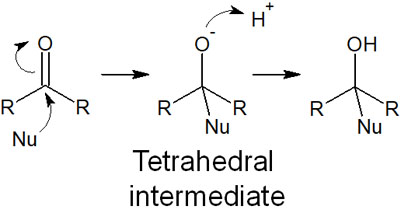
When does the nucleophile not attack the carbonyl carbons but rather the formation of enolates? The answer is when a bulky base is used.
In many cases, bulky bases are used in the formation of enolates. For example, LDA (Lithium diisopropylamide) is known as such a strong base. LDA is a compound with the following structure.
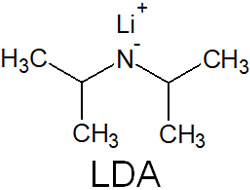
A bulky base has a large steric hindrance. Therefore, it cannot nucleophilic attack the carbonyl carbon.
Instead, it is possible to pull out the hydrogen atom attached to the alpha carbon of the carbonyl group. This is because proton withdrawal is less affected by steric hindrance.
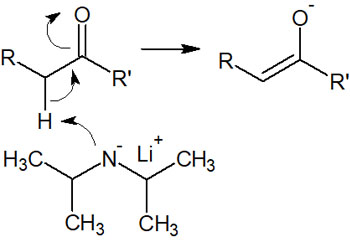
A bulky base also has the advantage that when an alkyl halide is added to a solution, it is difficult to react due to steric hindrance. Using a reagent that is a strong base but cannot make a nucleophilic attack is an important point in enolate formation.
-NaH (Sodium Hydride) Is Also Used as a Strong Base
Alternatively, NaH (sodium hydride) can also be used as a strong base to produce enolates. This is because although NaH is a strong base, it is not nucleophilic.
NaH is not a bulky base. Rather, it is a very small molecule. However, because of its small orbitals and lack of nucleophilicity, it is very important in enolate synthesis.
Electron-Withdrawing Groups, Such as Cyano and Nitro Groups, Can Be Used for Alkylation
It is not only ketones and aldehydes that produce anions by strong bases. Other electron-withdrawing groups can be used as enolate equivalents to synthesize compounds with anionic properties by strong bases.
Examples of electron-withdrawing groups include the following.
- Cyano group (-CN)
- Nitro group (-NO2)
- Ester (-COO-)
- Amide (=CO-NR2)
Because they are electron-withdrawing, the alpha carbon is highly acidic, as is the alpha carbon of the carbonyl group. Therefore, by adding a strong base, we can synthesize enolate equivalents with a carbanion. For example, it is as follows.
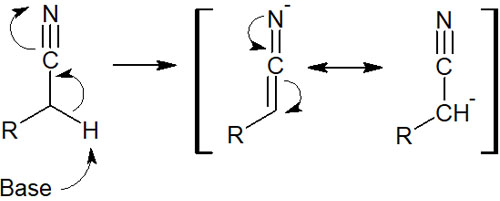
When an electron-withdrawing group is present in a molecule, the proton of the alpha carbon is pulled out, even if it is not a ketone or aldehyde.
Regioselectivity Is Important in Ketone Alkylation
There is one problem in the formation of enolates in compounds with electron-withdrawing groups, including ketones. That is regioselectivity. Which part of the α-hydrogen is pulled out and becomes anionic is important.
For example, in ketones, there are two places where the protons are pulled out, as shown below.
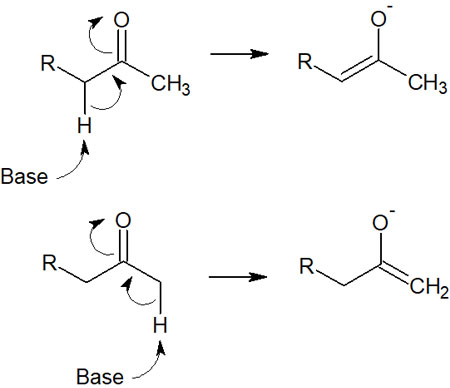
The reason why regioselectivity is so important in the use of enolate for alkylation is that two compounds may be formed. Therefore, we need to understand how enolates are synthesized.
Thermodynamic Control: Acidity and Alkene Stability Determine the Position of the Reaction
There are two ideas in the regioselectivity of enolates. One of them is thermodynamic control. In short, the lower the activation energy required to make a compound react, the more likely it is to react preferentially.
One of the most obvious thermodynamic control of regioselectivity is the difference in acidity. For the α-carbon of the carbonyl group, only one side will be alkylated if the acidity is different. For example, in the following compound with carbonyl groups on both sides of the α-carbon, the addition of a strong base will produce only one enolate.
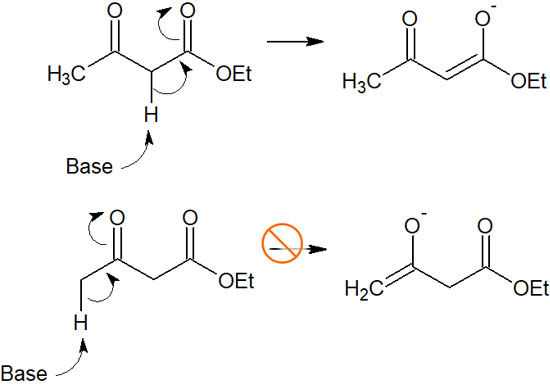
If two carbonyl groups are next to the α-carbon, it is easy to predict that the acidity will be higher. Therefore, due to regioselectivity in thermodynamic control, only one compound can be obtained.
-Compounds with Many Substituents Tend to Be Stable
However, compounds with carbonyl groups on both sides of the α-carbon are rare. So how can we predict which compounds will be generated?
When a compound makes a double bond, the compound is synthesized so that a polysubstituted alkene is formed. This is called the Saytzeff rule. There is an order of stability for alkenes, as shown below.

The double bond contains a π bond, which extends perpendicular to the bond. As a result, the π orbitals are parallel to the neighboring C-H bond, and the molecular structure is stabilized by weakly sharing electrons. This is called hyperconjugation.
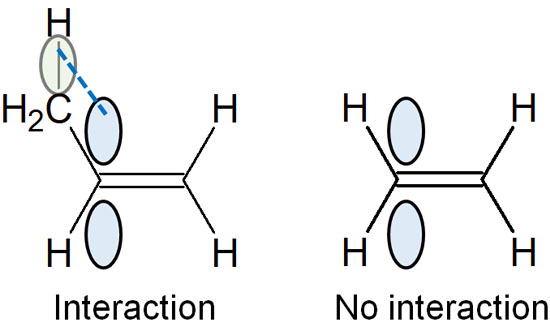
Therefore, the more substituents there are in an alkene, the more stable the alkene structure will be.
The same is true for enolate formation. When enolates are synthesized, intermediates with stable structures are preferentially formed. As a result, enolates are synthesized so that many alkyl chains are attached to the double bond.
For example, if we add NaH (sodium hydride), the enolate produced will look like this.
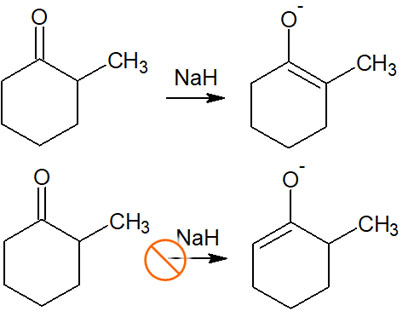
When considering regioselectivity in thermodynamic control, the Saytzeff rule allows us to predict the regioselectivity of the enolate.
Kinetic Control: Use of Bulky Bases to Synthesize Fewer Substituents
Is it possible to synthesize an enolate so that it becomes an alkene with fewer substituents? By using a bulky base, a compound can be synthesized to have fewer substituents.
Strong bases without steric hindrances, such as NaH, produce polysubstituted enolates due to regioselectivity caused by thermodynamic control, as explained earlier. On the other hand, when a bulky strong base such as LDA is used, a proton with few substituents is easily pulled out due to steric hindrance.
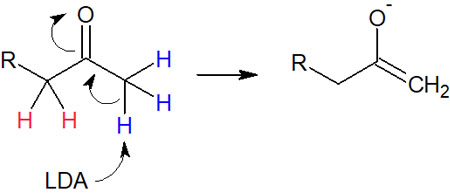
Considering the stability of polysubstituted alkenes, as mentioned above, enolates with more substituents are easier to produce in terms of activation energy. However, because of steric hindrance, when using a bulky base, the first proton to be withdrawn will be the one with fewer substituents.
This is the reaction condition under kinetic control. The greater the steric hindrance of the strong base used, the less substituent enolate will be synthesized. For example, the following synthesis proceeds under kinetic control.
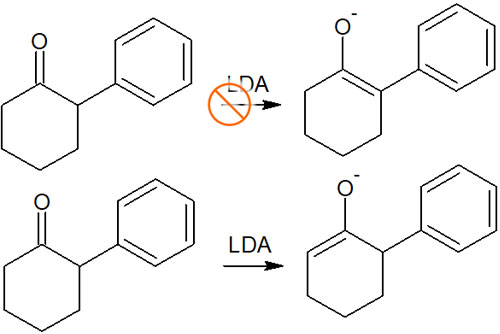
The regioselectivity of the enolate depends on the reagent used. When a small strong base such as NaH is used, the reaction proceeds under thermodynamic control. On the other hand, when a bulky strong base such as LDA is used, the reaction proceeds in the kinetic control due to steric hindrance.
Enolate Synthesis by Electron-Withdrawing Groups, Such as Ketones
The alpha carbon of the carbonyl group is known to be highly acidic. Therefore, enolates can be synthesized by using a strong base. Organic synthesis using enolates is very convenient because new alkyl chains can be synthesized through an SN2 reaction by adding alkyl halides.
In order to proceed with the reaction, we need to understand the difference between enol and enolate. You also need to learn what strong bases will allow you to synthesize enolates without the nucleophilic addition reaction occurring.
Another problem in the synthesis of enolates is regioselectivity: small bases, such as NaH, produce enolates with many substituents, while bulky bases, such as LDA, produce enolates with fewer substituents.
The reactions of enol and enolate are widely involved in important synthetic reactions in organic chemistry, such as Claisen condensation and aldol reaction. We have explained the basics of understanding these reactions, so please make sure you understand them beforehand.