Catalytic hydrogenation is a technique that can reduce compounds by a simple experimental procedure. Catalytic hydrogenation is also known as catalytic reduction.
Catalytic hydrogenation is a method of reacting with hydrogen (H2). Catalytic reduction causes hydrogen to be added to the compound, and the molecule’s shape is changed.
In catalytic reduction, the reagent used is almost always palladium on carbon (Pd/C). However, there are several types of reactions in catalytic hydrogenation. It is necessary to learn beforehand what kind of reduction reaction will occur for which functional group. This allows for the synthesis and deprotection of alkanes.
In this section, we will explain the synthesis method using catalytic reduction, including the reaction mechanism.
Table of Contents
- 1 Catalytic Hydrogenation with Palladium on Carbon (Pd/C)
- 2 Types of Synthetic Reactions Possible with Catalytic Reduction
- 2.1 Conversion of Alkenes (Double Bond) and Alkynes (Triple Bond) to Alkanes
- 2.2 Synthesis of cis (Z) Alkenes from Alkynes with Lindlar Catalyst
- 2.3 Use of Palladium on Carbons in Deprotection of Benzyl Groups
- 2.4 Reduction of Nitro Groups and Imines to Obtain Amines
- 2.5 Dehalogenation of the Benzene Ring
- 2.6 Reduction of Aldehydes and Ketones to Alcohols or Methylenes
- 3 Understanding the Reactions That Occur in Catalytic Hydrogenation
Catalytic Hydrogenation with Palladium on Carbon (Pd/C)
Compounds can be reduced by using a reducing agent. On the other hand, instead of using a reducing agent, using hydrogen and a metal catalyst to perform the synthesis is catalytic hydrogenation. The shape of the molecule is changed by the addition reaction with hydrogen.
Palladium is a metal catalyst that is frequently used in organic chemistry. Palladium on carbon is one of the palladium catalysts, and palladium on carbon (Pd/C) is a substance in which palladium is adsorbed on activated carbon.
In catalytic reduction, palladium on carbon is used mostly for synthetic reactions. In some cases, it is necessary to use another palladium catalyst, called a Lindlar catalyst, to synthesize alkenes (double bonds) from alkynes (triple bonds).
However, unless there is a specific reason, it is important to understand that we use palladium on carbon in catalytic hydrogenation.
Reaction Mechanism for Reduction of Compounds by Syn-Addition
What kind of reaction mechanism is used for catalytic reduction? The reduction reaction proceeds when a metal catalyst (palladium on carbon) is added and reacts with the compound in a hydrogen-filled condition.
In catalytic reduction, the synthetic reaction takes place at the surface of the palladium on carbon. Initially, hydrogen (H2) is adsorbed on the surface of the palladium on carbon. Subsequently, the compound coordinates with the palladium on carbon, which results in the addition of hydrogen to the molecule.
For example, in catalytic hydrogenation to a double bond, the reaction mechanism is as follows.
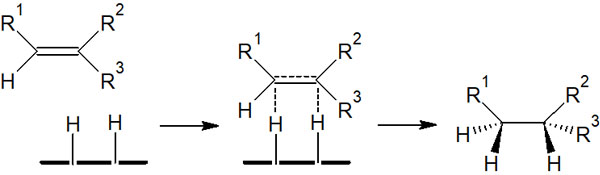
Since it reacts with the hydrogen atoms adsorbed on the metal, hydrogen will be added in the same direction. Synthesis proceeds by syn-addition, not by anti-addition.
Types of Synthetic Reactions Possible with Catalytic Reduction
What types of synthetic reactions are possible in catalytic reduction? The reaction mechanism is simple: hydrogen is added. However, there are many different types of synthetic reactions possible with catalytic hydrogenation, and each of these reactions must be learned.
The important synthetic reactions in catalytic hydrogenation are as follows.
- Alkenes and alkynes to alkanes
- Synthesis of alkenes with Lindlar catalyst
- Deprotection of benzyl groups
- Reduction of nitro groups and imines
- Synthesis of alcohols from aldehydes and ketones
It is essential to understand all of these reactions when performing catalytic hydrogenation. For example, if you want to convert a double bond to a single bond, and there is a nitro group in the molecule, the nitro group will be converted to an amino group.
In catalytic reduction, there is no steric hindrance because hydrogen is used for the reaction. Also, there is almost no selectivity for functional groups. Since any synthetic reactions that occur in catalytic hydrogenation proceed, it is necessary to understand what kind of reduction reactions take place.
Conversion of Alkenes (Double Bond) and Alkynes (Triple Bond) to Alkanes
A typical method of catalytic hydrogenation is the synthesis of alkanes. If there are double or triple bonds in the molecule, single-bonded compounds can be synthesized by catalytic reduction.
As mentioned above, there is no steric hindrance and no functional group selectivity. If alkenes and alkynes are present in the molecule, all multiple bonds can be converted to single bonds by catalytic hydrogenation.
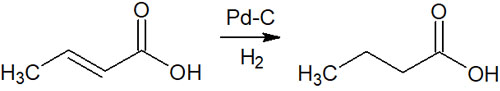
The reaction mechanism is not difficult; as mentioned earlier, hydrogen molecules are added through syn-addition. As a result, alkenes and alkynes are converted to alkanes.
When reducing an alkyne (triple bond), the synthesis cannot be stopped at the alkene (double bond) using palladium on carbon (Pd-C). Any multiple bonds present in the molecule are converted to alkanes (single bonds).
Synthesis of cis (Z) Alkenes from Alkynes with Lindlar Catalyst
In catalytic reduction, the synthetic reaction proceeds by regioselectivity through syn-addition. In that case, is it possible to react with alkynes to obtain alkenes (double bonds)?
As mentioned above, this is not possible when using palladium on carbon. Instead, it is possible to synthesize alkenes (double bonds) from alkynes (triple bonds) using a Lindlar catalyst.
The Lindlar catalyst also contains palladium as a catalyst. Instead of containing activated carbon, the Lindlar catalyst is a mixture of calcium carbonate (CaCO3) and palladium. Note that the addition of lead acetate or quinoline reduces the catalytic activity of the Lindlar catalyst.
Adding a substance to a catalyst to reduce its activity is called catalyst poisoning. Lead acetate and quinoline have a catalyst poisoning effect and can reduce the catalytic ability.
Why is it beneficial to weaken the catalytic activity by catalyst poisoning? It is because the weakness of the Lindlar catalyst slows down the synthesis of alkenes (double bonds) to alkanes (single bonds).
Therefore, using a Lindlar catalyst, alkenes can be synthesized from alkynes by syn-addition. All the compounds synthesized are cis (Z-form).
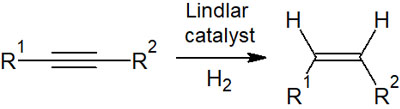
One way to obtain alkenes in a Z-selective manner, with no trans compounds (E) being formed, is to use a Lindlar catalyst. Although a strong catalyst will lead to an alkane, using a Lindlar catalyst, the synthetic reaction will stop at a compound with a double bond.
Use of Palladium on Carbons in Deprotection of Benzyl Groups
Catalytic reduction is also very important when you want to deprotection. This is because benzyl groups are frequently used as protecting groups for alcohols.
Alcohols are polarized and are known to be highly reactive functional groups. So, to prevent side reactions, the alcohol is protected by a benzyl group. If the alcohol is made into a benzyl ether, the reactivity of the hydroxy group (-OH) is eliminated, and no side reactions can occur.
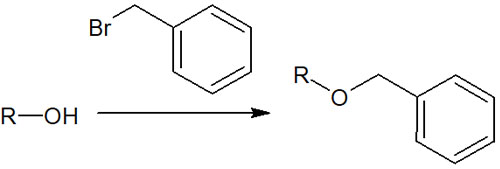
When the benzyl group is used as a protecting group, the method of deprotection is catalytic reduction. The benzyl group is removed by reacting a mixture of hydrogen and palladium on carbon (Pd/C).
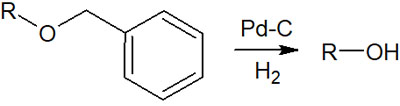
Benzyl groups have the advantage that the protecting group is difficult to remove under a variety of conditions, including acidic and basic. Deprotection of benzyl groups is difficult to achieve without catalytic reduction. On the other hand, the only option for deprotection is catalytic hydrogenation, and the lack of options for deprotection is a disadvantage.
Reduction of Nitro Groups and Imines to Obtain Amines
In the reduction of functional groups, a catalytic reduction is also used for nitro groups and imines. In particular, catalytic hydrogenation is frequently used to reduce nitro groups to yield amines.
There are several methods for reducing nitro groups and imines. Among them, catalytic hydrogenation is one of the most convenient methods. All you need to do is fill the hydrogen gas, add palladium on carbon, and stir. Moreover, work-up after the end of the reaction is simple.
By performing the catalytic reduction, a nitro group becomes an amino group, as shown below.
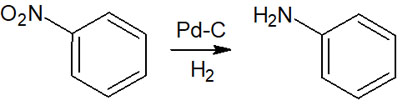
When reducing nitro groups and imines, a highly useful synthetic reaction is catalytic hydrogenation.
Dehalogenation of the Benzene Ring
Halogens bonded to the benzene ring are also reduced by catalytic reduction. Among the halogens, the fluorine atom (F) is not affected by the use of palladium on carbon. However, chlorine (Cl), bromine (Br), and iodine (I) atoms can be dehalogenated.
For example, catalytic reduction of chlorobenzene yields benzene, as shown below.
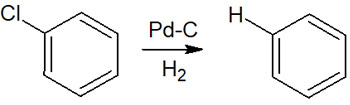
In any case, it is important to understand that Cl, Br, and I bonded to the benzene ring are replaced by hydrogen atoms through catalytic reduction.
-Tin Chloride (SnCl2) Does Not Reduce the Halogens on the Benzene Ring
How can we reduce without affecting the halogen on the benzene ring? In this case, we have to proceed with the synthesis by a different method than catalytic reduction.
For example, tin chloride (SnCl2) is known as a reagent that can reduce molecules. By using tin chloride, the halogen on the benzene ring is not reduced, but the other parts of the molecule can be reduced. For example, an amino group can be synthesized by reducing a nitro group without hydrogen substitution of the halogen, as shown below.
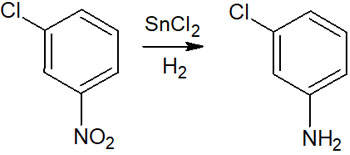
Catalytic reduction is frequently used because it is a simple method, but it is necessary to use different reduction methods depending on the compound.
Reduction of Aldehydes and Ketones to Alcohols or Methylenes
Compounds with aldehydes (formyl groups) and ketones (carbonyl groups) can also be reduced by catalytic hydrogenation. The reduction yields either an alcohol or a methylene compound.
Whether the resulting compounds are alcohols or methylenes depends on the type of palladium on carbon used and the reaction conditions.
There are different types of palladium on carbon (Pd/C). Therefore, depending on what type of palladium on carbon you use, you will get either alcohol or methylene.
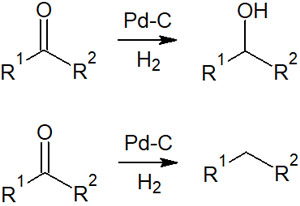
We will not explain the difference between each of them, as it is quite a niche. What is important is the fact that aldehydes and ketones can be reduced by catalytic reduction. To find out what kind of palladium on carbon can be used to make alcohol or methylene, please read the paper and do your own research before experimenting.
-Esters and Carboxylic Acids Cannot Be Reduced
The functional groups that are reduced in carbonyl compounds are aldehydes and ketones. Esters and carboxylic acids are not reduced by catalytic reduction.
If they have a C=O structure, they are not necessarily reduced to alcohols or methylene. It is important to understand that esters and carboxylic acids are not affected by reduction through catalytic hydrogenation.
In the case of esters and carboxylic acids, hydride reducing agents are used in the reduction reaction. The hydride reducing agent is a different reaction from the catalytic hydrogenation, and it is important to use a different reduction reaction depending on the compound you want to synthesize.
Understanding the Reactions That Occur in Catalytic Hydrogenation
One of the most frequently used organic synthesis reactions is catalytic hydrogenation. It is used in many situations, such as the synthesis of alkanes and deprotection. Palladium on carbon is mainly used, but when synthesizing alkenes from alkynes, a Lindlar catalyst is used.
All of the types of catalytic reduction that have been mentioned so far need to be memorized. This is because it is difficult to control only certain reactions.
For example, if there is a benzyl group and a nitro group in the molecule, both substituents will be reduced by catalytic reduction. To reduce only one of the substituents, an alternative synthesis method must be considered. Remembering the reduction reactions possible by catalytic hydrogenation will allow you to choose the best synthesis method.
One method of synthesis used in all organic chemistry laboratories is catalytic reduction. Try to use catalytic hydrogenation to obtain your target compounds.