In organic chemistry, we deal with a variety of molecules. One of the most important structures is conjugation. When double bonds (unsaturated bonds) and single bonds alternate, they are called conjugates.
Double bonds are known to undergo addition reactions. However, in conjugated dienes, two types of reactions occur when the addition reaction occurs; 1,2 addition and 1,4 addition. So you have to understand which reaction occurs.
One of the most famous reactions in 1,4 addition is Michael addition. For compounds with electron-withdrawing groups, the effect of 1,4 addition is especially important.
So we will explain the 1,2 and 1,4 addition to conjugated dienes and check the nature of the Michael addition for compounds with electron-withdrawing groups.
Table of Contents
Properties of Conjugated Dienes Stabilized by Resonance Structure
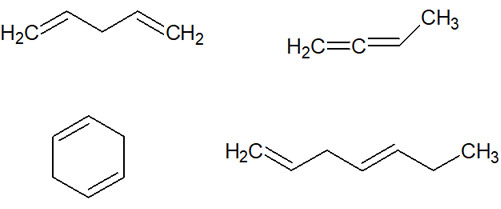
There are many compounds that have double bonds in the molecule. When an alkene has two alternating double and single bonds, it is called a conjugated diene. For example, the following compounds have a conjugated structure and are conjugated dienes because they have two double bonds.
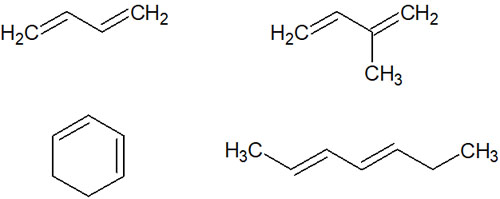
Two double bonds in a molecule do not necessarily make it a conjugated diene. The double bond and the single bond must alternate.
For example, the following compounds are not conjugated dienes.
So, among compounds with double bonds, why do we have to learn about molecules with conjugated structures separately? It’s because we can write resonance structures.
Even if an alkene has double bonds, if they are not conjugated, the double bonds have only general properties. On the other hand, if there is a conjugated structure, we can write resonance structures as follows.
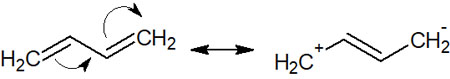
Because of the resonance, conjugated dienes have a more stable structure than non-conjugated dienes. They also have the property of double bonds, even if they are single bonds. In fact, the single bond in the middle of a conjugated diene has a shorter bond distance than an alkane and a longer bond distance than an alkene.
In conjugated structures, the π-electrons are dispersed to various places due to resonance, which makes the movement of electrons more complicated. This is a characteristic of compounds with a conjugated system.
Reactions of 1,2 Addition (Direct Addition) and 1,4 Addition (Conjugate Addition)
Why is the conjugated system of molecules so important? It’s because the movement of the π-electrons causes complex chemical reactions.
It is known that the presence of double bonds can cause addition reactions. This is called 1,2 addition (direct addition) because the molecule bonds to the first and second positions of the double bond. However, in conjugated dienes, not only 1,2 addition but also 1,4 addition (conjugate addition) occurs, which is an addition reaction at the 1 and 4 positions.
For example, when 1,3-butadiene reacts with HBr (hydrogen bromide), both 1,2 and 1,4 addition reactions occur, as shown below.
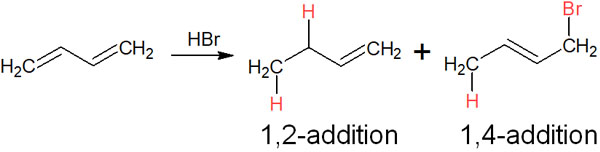
1,2 Addition (direct addition) is a typical addition reaction. In contrast, why does 1,4 addition (conjugate addition) occur? The reason is that the presence of a conjugated structure causes the molecule to resonate.
Reaction Mechanism of Addition Reaction to Conjugated Dienes
The reason for conjugate addition can be understood by checking the reaction mechanism of the addition reaction to conjugated dienes.
Initially, in the addition reaction, the π-electrons of the double bond attack the hydrogen atoms of the hydrogen halide. The result is the formation of a carbocation; in the case of 1,3-butadiene, the hydrogen atom is attached to the terminal carbon. This is because aryl cations are more stable than primary carbocations when considering the stability of carbocations.
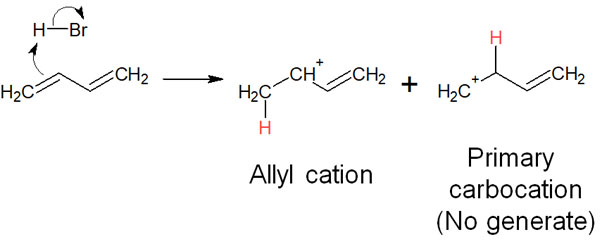
In the case of 1,2 addition (direct addition), the bromine ion attacks after the formation of the carbocation. As a result, the addition reaction is completed by the following reaction mechanism.
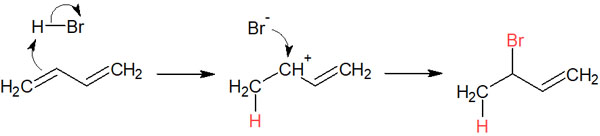
In contrast, in 1,4 addition (conjugate addition), after a hydrogen atom is bonded, the location of the double bond changes due to resonance. The bromine ion then attacks.
The reaction mechanism is as follows.
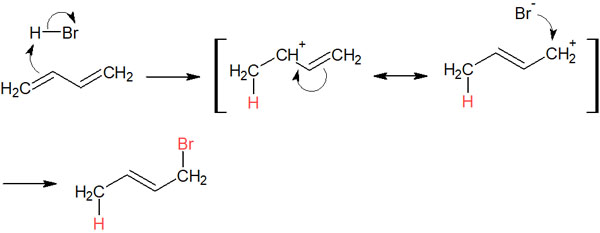
Because of the double bond next to the carbocation, electrons can be transferred. As a result, 1,4 addition can occur.
Kinetic and Thermodynamic Control Determines Regioselectivity
So which occurs preferentially, 1,2 addition or 1,4 addition? This depends on the reaction temperature. When the reaction is carried out at a low temperature (-78°C), 1,2 addition occurs. On the other hand, when the reaction is carried out at room temperature (e.g., 25°C), 1,4 addition occurs.
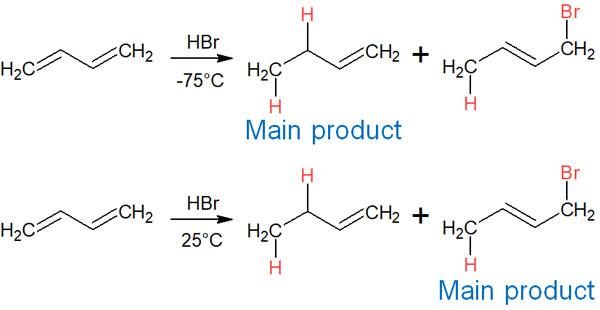
Under low-temperature conditions, 1,2 addition takes priority. On the other hand, under high-temperature conditions, 1,4 addition is preferred. Why does this difference occur?
-1,2 Addition Is Kinetic Control
The 1,2 addition in conjugated dienes is due to kinetic control. The kinetic control is based on the fact that the reaction takes place preferentially at low temperatures in a short time.
When a proton is added to the double bond, an aryl cation is formed. When the bromine ion attacks the carbocation, it is closer to the carbon atom in the second position than to the carbon atom in the fourth position. Therefore, if the temperature is lowered, thus lowering the movement of the molecule and the activation energy required for the reaction, the 1,2 addition will proceed preferentially.
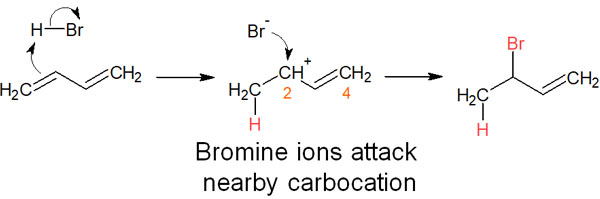
This is the reason why 1,2 addition is kinetic control, and the reaction proceeds preferentially at low-temperature conditions.
-1,4 Addition Is Thermodynamic Control
On the other hand, for 1,4 addition, the reaction is thermodynamic control. If the reaction is carried out at a high temperature and takes a long time, the reaction results in thermodynamic control. Thermodynamic control is the reaction that proceeds in such a way that a highly stable product is synthesized.
If the reaction temperature is high, stable compounds will be preferentially formed, even if the activation energy is somewhat high. For the addition reaction to a conjugated diene, when comparing the compounds formed, the stability differs as follows.
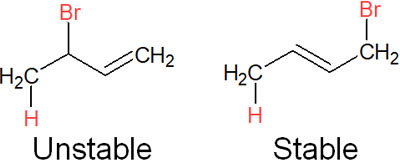
Why is there such a difference in stability between compounds? The allyl cation has a double bond. In molecules with a double bond, polysubstituted alkenes are known to be more stable.

This is explained by the phenomenon of hyperconjugation. The double bond has π electrons in it. Therefore, if there is a C-H bond next to it, the π electrons of the double bond and the C-H bond are parallel to each other. As a result, the structure is stabilized by weakly sharing electrons.
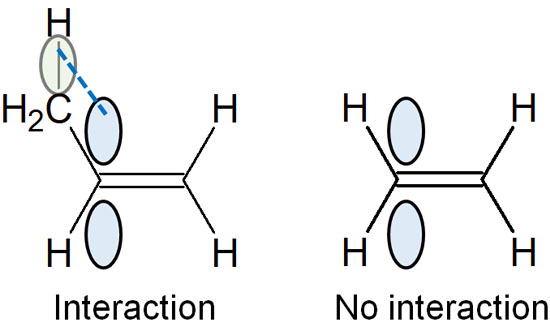
When addition reactions occur to conjugated dienes, the products are more stable with 1,4 addition. Since polysubstituted alkenes are preferentially synthesized, 1,4 addition (conjugate addition) proceeds under thermodynamic control at high reaction temperatures.
Michael Addition Is Important in α,β-Unsaturated Carbonyl Compounds
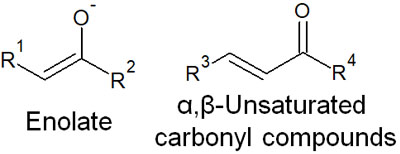
What is important in conjugated dienes is the fact that they cause 1,4 additions. In organic chemistry, there is another important addition, the Michael addition. The 1,4 addition to α,β-unsaturated carbonyl compounds is Michael addition.
In some carbonyl compounds, a carbonyl group (ketone) and a double bond exist next to each other in the molecule. These compounds are α,β-unsaturated carbonyl compounds. When a nucleophilic agent attacks α,β-unsaturated carbonyl compounds, two types of reactions occur.
- Nucleophilic attack on the carbonyl carbon
- Nucleophilic attack on the double bond (1,4 addition: Michael addition)
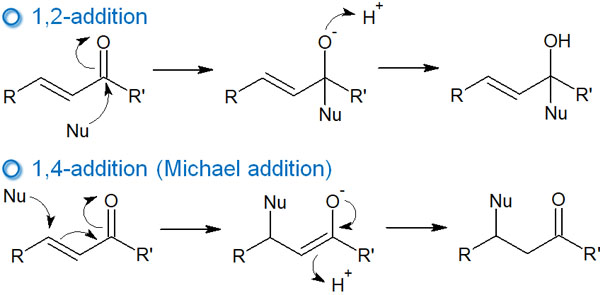
Thus, 1,2 or 1,4 addition occurs in α,β-unsaturated carbonyl compounds.
The way of thinking about which reaction occurs preferentially is the same as that of the conjugated dienes mentioned earlier. Due to the influence of the oxygen atom, the carbonyl carbon is positively polarized. Therefore, the activation energy is low, and 1,2 addition proceeds by kinetic control.
However, there is a large steric hindrance in attacking the carbonyl carbon. Nucleophilic attack on the double bond and the 1,4 addition provides a more stable compound. In other words, the Michael addition proceeds by thermodynamic control.
If the reaction is irreversible, the 1,2 addition proceeds by lowering the activation energy. On the other hand, in the case of a reversible reaction, the equilibrium reaction takes place when the reaction is carried out for a long time, and the reaction proceeds in such a way that a stable compound is finally synthesized even if the reaction rate is slow.
-Electron-Withdrawing Groups Such as Cyano and Nitro Groups Cause Michael Addition
If there is an electron-withdrawing group, Michael addition is carried out. A typical example of an electron-withdrawing group is a ketone. Therefore, α,β-unsaturated carbonyl compounds are often used as examples of Michael addition.
However, ketones are not the only electron-withdrawing groups. Cyano and nitro groups are also known to be electron-withdrawing groups. When these functional groups are present next to a double bond, the reaction can proceed by Michael addition.
In addition to alkenes, Michael addition can also occur when an electron-withdrawing group is present on an alkyne (triple bond).
Thermodynamic Control of Enolate Synthesis: Retro-Aldol Reaction
Nucleophilic attack by strong bases on α,β-unsaturated carbonyl compounds generally results in 1,2 addition. This is because the acid-base reaction allows the synthesis to proceed more quickly. In fact, Grignard reagent, known as a strong base, makes 1,2 additions to α,β-unsaturated carbonyl compounds.
On the other hand, when enolate is used, the reaction proceeds by thermodynamic control, and Michael addition takes place. In the case of Michael addition using α,β-unsaturated carbonyl compounds, the synthetic reaction using enolate is very important.
Why do enolates and α,β-unsaturated carbonyl compounds undergo Michael addition instead of 1,2 addition? It is because the reaction between enolate and α,β-unsaturated carbonyl compounds is a reversible reaction.
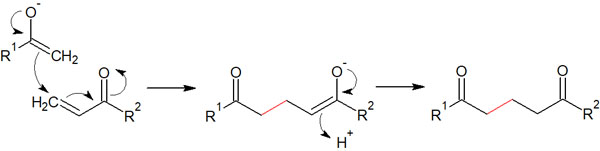
When an enolate reacts with a ketone (or aldehyde), it is called an aldol reaction. The aldol reaction allows for the creation of a new carbon bond. However, even if the product is obtained by the aldol reaction, in some cases, it may return to the original compound. This is called retro-aldol reaction.
By 1,2 addition (direct addition), enolate can attack the carbonyl carbon. However, the product of 1,2 addition is more unstable than the product of 1,4 addition (conjugate addition). Therefore, the retro-aldol reaction leads to the formation of stable Michael addition compounds with the passage of time.
Due to the equilibrium reaction caused by steric hindrance, the reaction of enolates with α,β-unsaturated carbonyl compounds proceeds under thermodynamic control. Therefore, using enolates, the following compounds can be obtained by conjugate addition.
One of the important questions in organic chemistry is whether 1,2 addition or 1,4 addition (Michael addition) occurs.
In a nucleophilic attack on an α,β-unsaturated carbonyl compound, both 1,2 and 1,4 addition can occur. However, depending on the reaction conditions, which of the two is preferred will vary. Therefore, it is necessary to learn when Michael addition is preferred.
Conditions and Reaction Mechanism for 1,2 and 1,4 Addition
When double bonds exist alternately, it is called a conjugated structure. In these compounds, a special reaction occurs: not only 1,2 addition, but also 1,4 addition occurs in conjugated dienes.
It is important to learn the difference between 1,2 and 1,4 additions. In kinetic control, 1,2 addition occurs, and in thermodynamic control, 1,4 addition occurs.
The same can be said for Michael addition to α,β-unsaturated carbonyl compounds. In the case of a reversible reaction rather than an irreversible reaction, Michael addition is preferred. A frequently used example is the aldol reaction between enolate and α,β-unsaturated carbonyl compounds.
Some molecules have multiple reaction sites. One of them is a compound with a conjugated structure. Let’s understand how 1,2 or 1,4 addition occurs.