Synthetic reactions that create carbon bonds are very important in organic chemistry. Among those reactions, the aldol reaction is one of the most frequently used synthetic reactions. The aldol reaction is the synthetic reaction that all of us learn when we study organic chemistry.
However, the aldol reaction doesn’t end with a single reaction. After the product is formed, the reaction may proceed further by dehydration. This is called aldol condensation.
The aldol reaction is an important reaction, and we need to understand the reaction mechanism in detail. Otherwise, we cannot predict what kind of products can be synthesized.
Many studies have been done on the aldol reaction. Therefore, there are many applications. This section explains the basics of the aldol reaction and aldol condensation.
Table of Contents
The reaction of Enolates with Ketones or Aldehydes Are Aldol Reactions
For compounds with carbonyl groups, keto-enol tautomerism is possible. Normally, the molecule is in the keto form, but it can change its shape to the enol form.
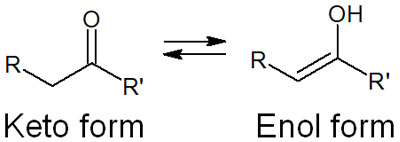
Whether or not it can become an enol form involves the α-carbon of the carbonyl group (the carbon atom next to the carbonyl group). The hydrogen atom (α-hydrogen) bonded to the carbon atom next to the carbonyl group is highly acidic and can easily be pulled out by a base. A result is an enol form.
The molecule produced in this process is called an enolate. The proton is withdrawn, and the enolate is formed as follows.
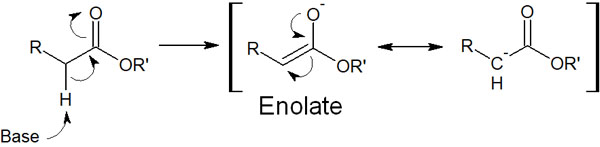
In enolates, the α-carbon is negatively charged. Due to its carbanionic nature, it is a nucleophile. Therefore, it can attack the carbonyl carbon of ketones and aldehydes.
The enolate reacts with the carbonyl compound to yield a new product (β-hydroxycarbonyl compound), as shown below.
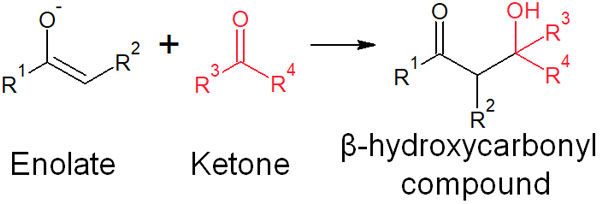
This reaction, in which β-hydroxycarbonyl compounds are synthesized, is called the aldol reaction. Since it is an addition reaction, it is also called aldol addition.
-Difference from Claisen Condensation
The Claisen condensation is a chemical reaction similar to the aldol reaction. The Claisen condensation is a synthetic reaction of enolates and esters. In addition, unlike the aldol reaction, the ester is removed in the Claisen condensation. It is the same reaction with enolate, but the reaction mechanism is different.
If the enolate reacts with a ketone or aldehyde, the reaction is an aldol reaction. On the other hand, when enolate reacts with an ester, the reaction is a Claisen condensation. It is important to understand these differences.
Reaction Mechanism of Aldol Addition by Base
How does the aldol reaction proceed? In general, aldol addition proceeds by base catalysis. The formation of enolate by the base causes the aldol reaction to occur.
The reaction mechanism is as follows.
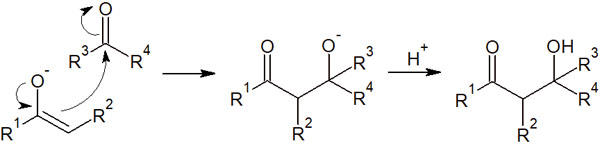
As mentioned above, the alpha carbon is negatively charged. Therefore, it can attack the carbonyl carbon of ketones and aldehydes. As a result, a new carbon chain can be created.
-The Reaction Proceeds with a Catalytic Amount of Base
Note that in aldol addition, the synthesis proceeds with a catalytic amount of base. It is not required to add an equivalent amount of base. Therefore, even if a weaker base than enolate is used, the aldol reaction will proceed.
What is the reason for this? The entire reaction mechanism of aldol addition can be described as follows.
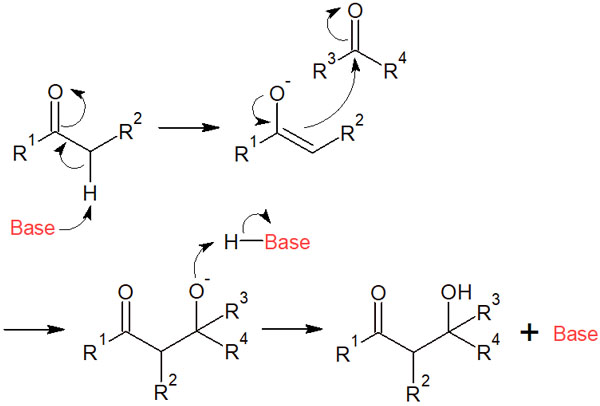
Initially, the base pulls out a proton to form an enolate. Then, as the aldol addition reaction proceeds, the H+ bound to the base is pulled out, and the base is regenerated. Therefore, the base pulls out the alpha hydrogen again to make enolate.
This is the reason why the reaction proceeds if there is a catalytic amount of base in aldol addition. Incidentally, one equivalent amount of base is required for the Claisen condensation. The reason for this is that the base does not regenerate as in the aldol reaction.
Crossed Aldol Reactions Are Less Useful
In aldol addition, aldol reactions between the same molecules are frequently used as an example. Is it possible to make an aldol reaction using different molecules? The aldol reaction between two different molecules is called a crossed aldol reaction.
In general, however, crossed aldol reactions are not useful. The reason for this is that many compounds are formed.
For example, is it possible to synthesize the following compound by adding propionaldehyde after acetaldehyde is enolated with a base?
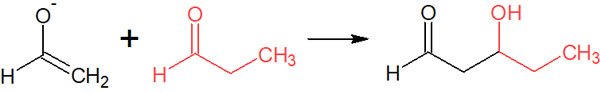
The answer is that you cannot synthesize only this compound. Many other products can be synthesized as well.
As mentioned earlier, the α-hydrogen of the carbonyl group is highly acidic. Since enolate is also a strong base, enolate pulls out the protons bound to the α-carbon of propionaldehyde. As a result, enolate derived from propionaldehyde is also synthesized.

Thus, two types of enolates are formed in the solution. Therefore, the following four types of reactions occur in solution.
- The reaction of acetaldehyde (enolate) with acetaldehyde
- The reaction of acetaldehyde (enolate) with propionaldehyde
- The reaction of propionaldehyde (enolate) with propionaldehyde
- The reaction of propionaldehyde (enolate) with acetaldehyde
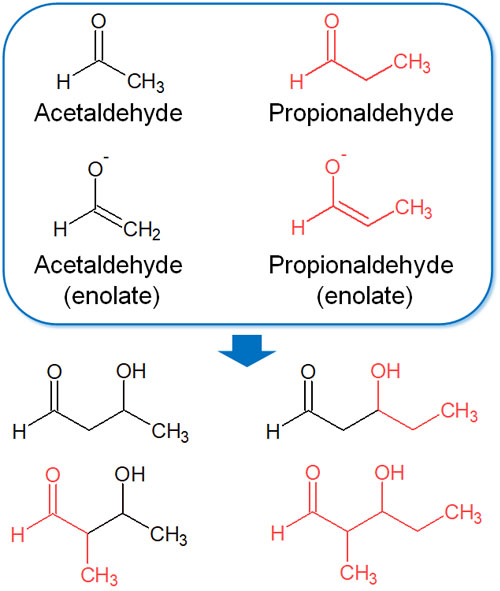
Four different compounds are synthesized, as shown in the figure above. The crossed aldol reaction is less useful because several byproducts are synthesized. Because of the complexity of the reaction, there are few cases where different compounds are used in the aldol reaction.
Crossed Aldol Reaction Should Be Performed with a Compound Without α-Hydrogen
Is the crossed aldol reaction meaningless? No, crossed aldol reactions are meaningful under certain conditions. This is when aldol addition is performed using a compound that has no α-hydrogen.
In some compounds, there are no hydrogen atoms in the alpha carbon. In that case, no acid-base reaction occurs when reacting with enolate. The synthetic reaction can proceed without the formation of other enolates in the solution.
For example, the following compounds react with each other.
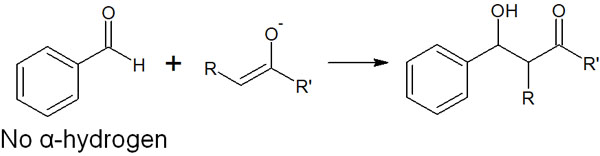
The compound shown in the above figure, which reacts with enolate, has no α-hydrogen. Therefore, no acid-base reaction will occur; only one reaction will take place. To succeed in the crossed aldol reaction, it is important to understand that the compounds to be reacted are limited.
As for what form of enolate can be synthesized, the regioselectivity can be controlled by the type of base; if the steric hindrance is small, such as NaH, an enolate with many substituents is synthesized. On the other hand, in the case of a base with high bulk, such as LDA, an enolate with few substituents is synthesized due to steric hindrance.
The desired compound can then be obtained by the crossed aldol reaction by adding a carbonyl compound that does not have α-hydrogen.
Aldol Condensation Proceeds in the E1cB Reaction (Elimination Reaction)
When performing aldol addition, a small amount of base is added to obtain aldol compound (β-hydroxycarbonyl compound). By adding a catalytic amount of base, the target compound can be obtained while generating enolate.
On the other hand, if the amount of base is high, different compounds can be synthesized. Specifically, alkene compounds can be obtained through elimination reactions.
Normally, alcohols do not undergo elimination. However, if -OH (hydroxy group) is present at the β-position of the ketone (carbonyl group), the hydroxy group is released after the compound is enolated. The reaction mechanism is as follows.
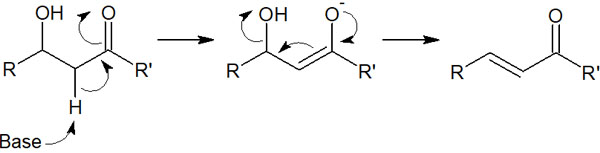
This is called the E1cB reaction. If a ketone is present, the compound becomes an enolate because the alpha hydrogen is highly acidic. Then when the electrons return, if there is a leaving group, the elimination reaction occurs, creating a double bond.
The removal of water or alcohol to form a new molecule is called condensation. Starting from the aldol reaction, the E1cB reaction leads to the elimination of -OH, and this reaction is called aldol condensation, as mentioned above. In addition to aldol addition, the condensation reaction proceeds in aldol condensation.
Incidentally, in general, aldol condensation tends to progress under extreme conditions, such as the use of strong bases, high reaction temperatures, and long reaction times.
-Crossed Aldol Condensation Is Available
As for aldol condensation, crossed aldol condensation is also available. When performing crossed aldol reactions, if the base is in excess, the E1cB reaction proceeds, and crossed aldol condensation occurs.
The amount of base should be adjusted according to whether you want to obtain an aldol compound or an alkene by crossed aldol condensation.
Dehydration by E1 or E2 Elimination Occurs in Acid Catalyst (Acidic Conditions)
As for aldol condensation, it proceeds under acidic conditions as well as basic conditions. The fact that alkenes (double bonds) are formed by the elimination reaction is the same. However, the reaction mechanism is different from that of basic conditions. With the use of an acid catalyst, dehydration occurs by E1 or E2 elimination.
Under acidic conditions, a proton binds to an oxygen atom, which gives it a positive charge. Water is then released, and a double bond is formed.
For example, the reaction mechanism of dehydration by E2 elimination using an acid catalyst is as follows.

Since the reaction is carried out under acidic conditions, there are very few bases present in the solution. However, since α-hydrogen is highly acidic, the E2 reaction proceeds even with a small amount of bases. In addition, the reaction may proceed in the E1 reaction.
Cyclization in Intramolecular Aldol Reactions
If the molecule has a ketone (carbonyl group) or aldehyde (formyl group) in the molecule, it will be cyclized. This is called intramolecular aldol reaction.
The reaction mechanism is the same as the aldol addition described above. Let’s understand that an aldol reaction occurs within the same molecule. In this case, compounds with five or six-membered rings are mainly synthesized. For example, in the following compounds, the addition of a base causes the aldol reaction (aldol condensation) to proceed.
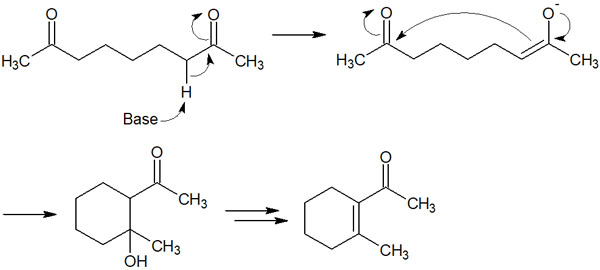
What is important is the formation of the six-membered ring compound.
In the carbonyl compound shown above, there are two types of α-carbons. However, the enolate can only attack at one place. For example, in the following reaction mechanism, an eight-membered ring compound is formed.
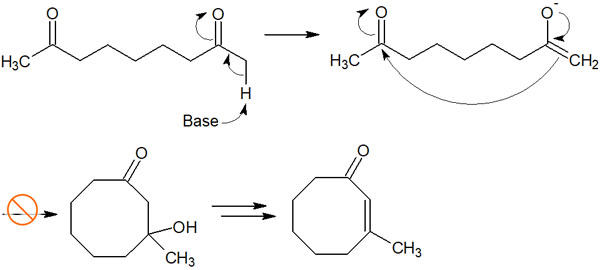
However, it is not possible to synthesize 8-membered ring compounds due to their high strain. Only compounds with 6-membered rings, which have less strain, can be obtained by intramolecular aldol condensation.
In aldol addition and aldol condensation, it is also important to consider regioselectivity. For intramolecular aldol condensation, compounds with 5 or 6-membered rings can be obtained.
Aldol Reactions to Create Carbon Bonds
When studying organic chemistry, the aldol reaction is what everyone studies. The reaction of enolates with ketones (or aldehydes) is an aldol reaction.
The first thing to understand is the aldol addition. The enolate attacks the carbonyl carbons to give a β-hydroxycarbonyl compound. 1 equivalent amount of base is not needed; aldol addition proceeds with a catalytic amount of base.
However, if the amount of base is high, elimination reactions will occur. Dehydration results in the formation of compounds with alkenes (double bonds). Aldol condensation can also occur even with an acid catalyst, yielding an alkene.
Although the aldol reaction is an important reaction to create carbon bonds, the product varies depending on the reaction conditions and the base used. So let’s understand how the reaction can be made to obtain the desired compound.