One way to create a new carbon chain is through synthetic reactions using enolates. One of the organic chemical syntheses with enolates is Claisen condensation.
In Claisen condensation, molecules with esters react with enolates. As a result, new carbonyl compounds can be obtained. When Claisen condensation occurs intramolecularly, it is called Dieckmann condensation (intramolecular Claisen condensation).
One of the most important organic chemical reactions is Claisen condensation. However, in the case of the Claisen condensation, there are several points that need to be understood beforehand, such as the base to be used and the reaction mechanism.
In order to obtain the target compound, you must select the right reagents and consider the reaction conditions. In this section, we will explain the reaction mechanism of Claisen condensation and some important points to keep in mind.
Table of Contents
The Reaction Between Enolate and Ester Is the Claisen Condensation
The condensation reaction of two esters is the Claisen condensation. The Claisen condensation is one of the synthetic reactions using enolates.
In compounds with a carbonyl group, including esters, the hydrogen atom (alpha hydrogen) of the alpha carbon is easily pulled out by the base. The result is a change from a keto form to an enol form compound.
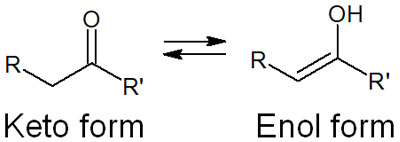
The base makes the molecule into an enol form. The enolate is an enol compound produced by the base. By adding a basic reagent to the ester, enolate can be synthesized as follows.
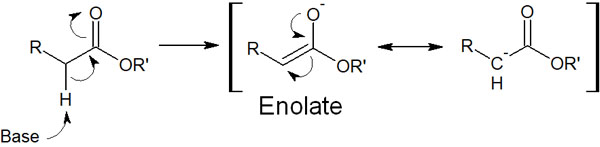
The carbon atoms of enolate are negatively charged. Because it is a carbanion, enolate is highly nucleophilic. Therefore, it can attack other carbonyl carbons and create new carbon bonds.
Enolate is a nucleophile that attacks esters by the following reaction mechanism.
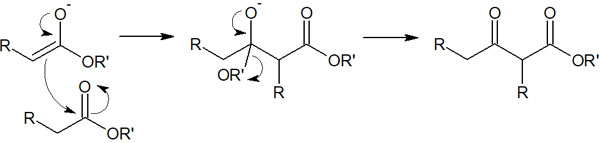
Carbonyl carbons are known to be susceptible to nucleophilic attack. Therefore, the reaction proceeds when the enolate makes a nucleophilic attack on the ester.
Then, when the electrons in the oxygen atom return, the ester is released. The removal of water or alcohol to form a new molecule is called condensation. This reaction is a type of condensation and was discovered by the German chemist Claisen, hence the name Claisen condensation.
-What Is the Difference Between the Aldol Reaction?
A synthetic reaction similar to Claisen condensation is the aldol reaction. In the same way, the aldol reaction proceeds with the enolate.
In Claisen condensation, enolate reacts with esters. In the aldol reaction, on the other hand, enolate reacts with ketones or aldehydes. Depending on whether the compound reacting with enolate is an ester or a ketone (or aldehyde), the name of the synthetic reaction and the products change.
Note that one equivalent amount of base is required for Claisen condensation. The aldol reaction, on the other hand, requires only a catalytic amount of base to proceed.
β-Keto Esters Become Enolates by Acid-Base Reactions
Are ketones not attacked by enolates after the formation of compounds by Claisen condensation?
The compounds synthesized by Claisen condensation are called β-keto esters. Ketones have a higher electrophilic property than esters. Therefore, it would seem that enolate makes a nucleophilic attack on the ketones of the β-keto ester, not the ester.
However, in fact, the product ketone (β-keto ester) does not react with the enolate. This is because the α-carbon of the β-ketoester is highly acidic.
Claisen condensation is a synthetic reaction under basic conditions. The presence of a strong base results in the formation of enolates. Because of the presence of a base in the solution, the hydrogen atom (α-hydrogen) bonded to the α-carbon of the β-keto ester is pulled out by the base. As a result, the following stable enolate ions are formed.
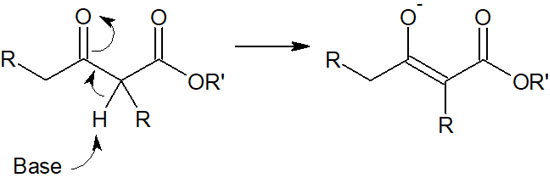
As a result of the change in form from the β-keto ester by the base, it is no longer subject to nucleophilic attack by enolate. Therefore, in Claisen condensation, the β-keto ester does not react with the enolate.
When the reaction is stopped, an acidic solution such as hydrochloric acid is added. After the acid-base reaction, the enolate returns to the form of the β-keto ester.
Cyclization by Intramolecular Condensation Is Called Dieckmann Condensation
When two esters exist in a molecule, they are cyclized by intramolecular Claisen condensation. This reaction is called Dieckmann condensation.
The reaction mechanism is exactly the same as that of Claisen condensation. However, the name of the reaction changes depending on whether the condensation occurs between two ester molecules or within a molecule.
The conditions for the Dieckmann condensation to occur are when the compound formed by cyclization is a five- or six-membered ring. In the case of four- or seven-membered rings, the reaction almost never proceeds because of the high strain on the stereochemistry. Understand that in the Dieckmann condensation, the product will be a five- or six-membered ring.
If these conditions are met, Dieckmann condensation proceeds by the following reaction mechanism.
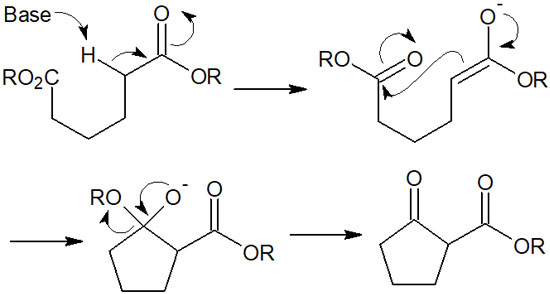
If you understand the reaction mechanism of Claisen condensation, Dieckmann condensation is easy to understand. Under base conditions, β-keto esters can be synthesized by cyclization in intramolecular reactions.
-Alkylation with Alkyl Halides Is possible
In addition, as mentioned above, the β-keto ester exists as a stable enolate because it is reacted under basic conditions. Therefore, it can be alkylated by reacting with alkyl halides before adding the acid.
For example, the following.
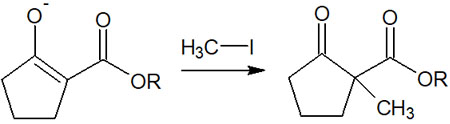
Even though the reactivity is lower because it is a stable enolate, it still has nucleophilic properties because the carbon atom is negatively charged. Therefore, after proceeding with the reaction by Claisen condensation (or Dieckmann condensation), it is possible to create a carbon chain by alkylation.
Crossed Claisen Condensation, Which Occurs in Two Different Molecules
In Claisen condensation, the chemical reaction is explained by the example of Claisen condensation using the same molecules. Can’t we use two different molecules for Claisen condensation? The synthesis of Claisen condensation with different molecules is called crossed Claisen condensation.
In crossed Claisen condensation, the following reactions are used.
- The reaction of esters with esters
- The reactions of esters with ketones
As for ketones, they are also known to produce enolates. Therefore, in addition to crossed Claisen condensation between esters, it is also possible to react with an ester and a ketone. For example, the reaction mechanism for an ester and a ketone is as follows.
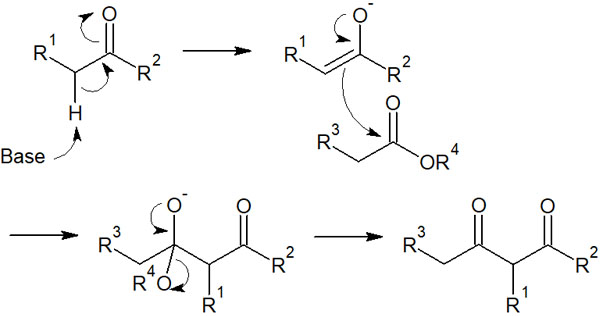
In the Claisen condensation with two esters, β-keto esters are synthesized. On the other hand, crossed Claisen condensation of esters and ketones allows the synthesis of β-diketone compounds.
Crossed Claisen Condensation Is Effective If One Compound Does Not Have a Hydrogen Atom at the α-Position
There is a point of caution when performing crossed Claisen condensation. That is to use an ester without α-hydrogen.
To get an enolate, the molecule must have alpha hydrogen; the proton attached to the α-carbon is pulled out to synthesize the enolate. However, if both compounds to be reacted have α-hydrogen, various kinds of enolates are produced, and the reaction is complicated.
Therefore, it is necessary to react to esters so that the base pulls out only one alpha hydrogen. For example, in the following reaction between esters, there is only one α-hydrogen.
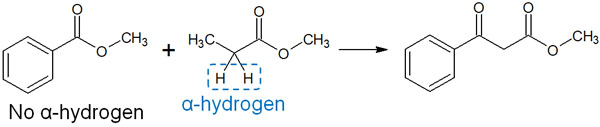
Crossed Claisen condensation is useful when one ester (or ketone) has no alpha-hydrogen in it. If there are several α-hydrogen atoms in the compound to be reacted, the reaction will be complicated. Therefore, the crossed Claisen condensation is limited in the conditions of the reaction.
With the Use of Sodium Hydroxide, It Becomes Carboxylic Acid and Can Decarboxylation
The use of sodium hydroxide (NaOH) as a base used in Claisen condensation is rare.
It is possible to synthesize enolate by using sodium hydroxide. Nevertheless, why is NaOH rarely utilized in Claisen condensation? The reason for this is that OH– attacks the ester, which results in the synthesis of carboxylic acids.
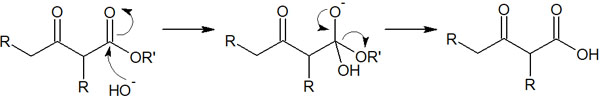
In this way, compounds with a carbonyl group (ketone) in the third position of the carboxylic acid are synthesized.
Incidentally, a carboxylic acid with a ketone at the 3-position is decarboxylated when heated. The compound is decomposed, and the carboxylic acid is released as carbon dioxide. The reaction mechanism for decarboxylation is as follows.
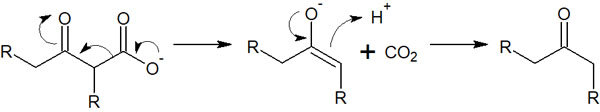
If you want to obtain a carboxylic acid or perform decarboxylation after the Claisen condensation, you can use sodium hydroxide. But otherwise, NaOH will not be used as a base.
Transesterification with Sodium Methoxide and Sodium Ethoxide
So, is it enough to use any other base? For example, sodium methoxide and sodium ethoxide are known to be bases.
However, there are few opportunities to use alkoxide as a strong base. This is because when sodium methoxide or sodium ethoxide is used, methoxide or ethoxide are formed through transesterification. It is as follows.
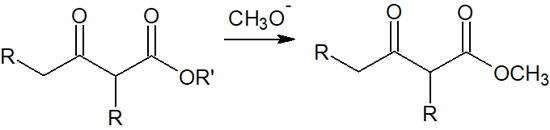
It is widely known that adding an alcohol to an ester causes transesterification. Because of the transesterification occurring, we rarely use sodium methoxide or sodium ethoxide in bases.
-The Use of LDA or NaH Is Common
Then, what kind of bases are used in Claisen condensation? Bases such as LDA (lithium diisopropylamide) and NaH (sodium hydride) are frequently used in the synthesis of enolates, not only in Claisen condensation but also in other processes.
LDA is a bulky base, as shown below.
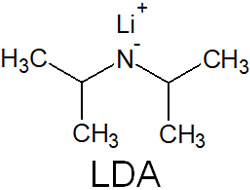
Due to its large steric hindrance, LDA has almost no nucleophilic properties and acts only as a base. NaH is also a strong base with no nucleophilic properties. When synthesizing enolates, we should understand the differences in the properties of the bases and using the most appropriate base.
Use of Enolates and Claisen Condensation
When making new carbon bonds in organic chemistry, chemical reactions that utilize enolates are important. There are several synthetic reactions using enolates, such as aldol reaction and Michael addition, and one of them is the Claisen condensation.
One difference from other synthetic reactions is the use of esters. The synthetic reaction using enolates and esters is Claisen condensation. In addition, cyclization by intramolecular Claisen condensation is called Dieckmann condensation.
In Claisen condensation, crossed Claisen condensation, which uses different molecules, is also possible. However, the situations where it can be used are limited, such as when using molecules that do not have α-hydrogen.
In addition, let’s consider the bases to be used. Some bases cause side reactions, so you need to select the most suitable base to obtain the target compound. By understanding these, you can obtain the target compound through Claisen condensation.