An important element in organic chemistry is optical isomers (chiral molecules). They are also called chirality, and even though they may appear to have the same structural formula, the presence of a chiral center results in a mixture of completely different compounds.
In organic chemistry, optical isomers must be clearly separated because they are completely different compounds. This is because having a chiral center changes the properties of the compound. Also, it is difficult to synthesize optical isomers separately.
So how do we think about chiral compounds?
The chiral carbon atoms have to be considered in stereochemistry. Here we will explain the basics of the properties and concepts of chiral compounds.
Table of Contents
Chiral Carbon Atoms Make Chiral Compounds
Four substituents can be attached to one carbon atom. If all four substituents are different, the compound is an optical isomer. If there is a chiral carbon atom, the compound is called a chiral compound.
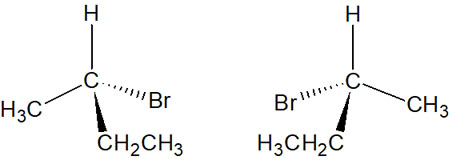
The two compounds that are in an optical isomeric relationship are called enantiomers.
The relationship between the right and left hands is frequently used as an example when explaining the chiral center. The right and left hands are symmetrical to each other as if reflected in a mirror. The same is true for optical isomers, which are symmetrical when reflected in a mirror.
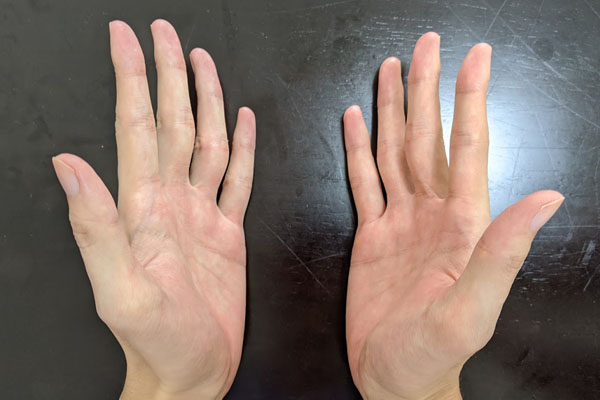
However, the right hand and the left hand cannot be completely superimposed on each other. It is impossible to try to create the same shape, as shown below.
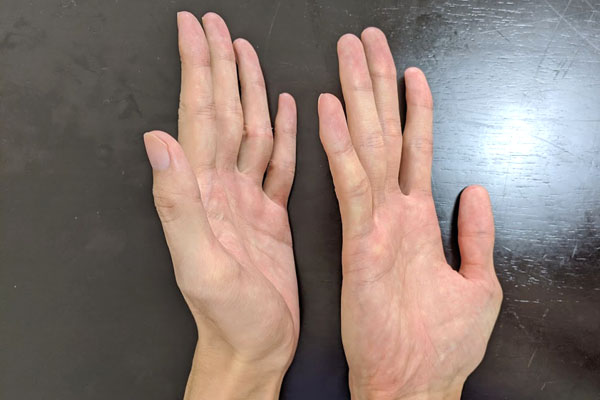
You have to realize that enantiomers have the same structural formula but are completely different compounds. By the way, a compound with a chiral center has chirality. On the other hand, if it is not a chiral compound, it is called an achiral.
Chirality is considered when there are four different substituents attached to a carbon atom. Therefore, in double or triple bonds, there is no chirality. Of course, in a double bond, cis-trans isomers must be considered. Cis-trans are different isomers than optical isomers, and they are not the same isomer.
Notations Are Fischer Projection and Dashed-Wedge Notation
How should these chiral compounds be described? For compounds with chiral centers, there are two ways to describe them. One is the Fischer projection, and the other is the dashed-wedge notation.
The Fischer projection is the way to describe the molecule in planar form, even if it has a chiral center. On the other hand, in dashed-wedge notation, the location of substituents is described in three dimensions.
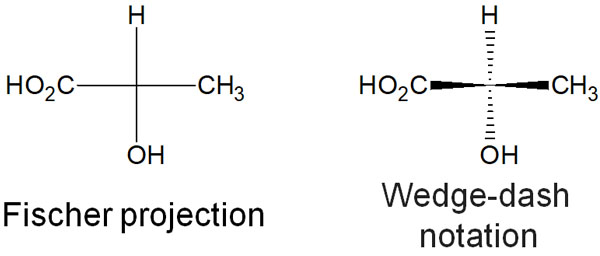
For compounds with chirality, both methods are widely used. Therefore, you can use either method.
In general, however, the dashed-wedge notation is widely used. This is because the dashed-wedge notation is more accurate in describing the molecular structure.
Specific Enantiomeric Synthesis Is Difficult and Results in Racemic Mixture
Why is it important to distinguish optical isomers in organic chemistry? It’s because different enantiomers have different properties.
The famous compound is thalidomide. In the past, thalidomide was sold as a sleeping pill. However, it has chirality, and while one has a sleeping effect, the other has a teratogenic effect on the fetus. As a result, it caused drug damage.
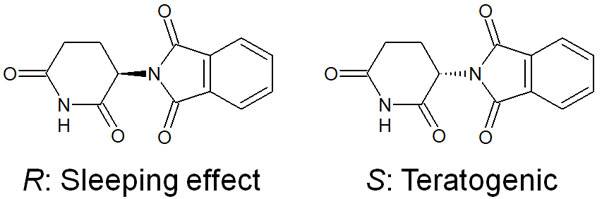
Each enantiomer has completely different bioactivity (i.e., functions in the body). Nevertheless, it is very difficult to synthesize only one of the enantiomers in organic chemistry. It is difficult to obtain one enantiomer (an optically active compound) selectively, and in most cases, it is racemized.
When two chiral compounds are mixed together equally, they are called racemic mixtures. With a chiral center, it is difficult to avoid the racemization of the compound.
-How to Separate Chiral Compounds
How can we get only one enantiomer? There is a way to do this by using a catalyst with chirality. Instead of an ordinary catalyst, a catalyst with chiral activity is used to obtain a specific enantiomer.
By using a special catalyst, only one enantiomer may be obtained instead of a racemic mixture.
Alternatively, the enantiomers can be separated by chromatography. Even when synthesized using a chiral active catalyst, obtaining a single enantiomer requires a high level of knowledge and skill. Therefore, chiral adsorbents are used to separate the compounds.
Typical column chromatography does not allow for the separation of racemic mixtures. On the other hand, by using a chiral stationary phase (chiral column) and performing column chromatography, a mixture of enantiomers can be separated.
Meso Compound Have a Chiral Center but No Optical Activity
Is it always an optical isomer if there is a chiral center? In this regard, the presence of a chiral carbon atom does not always mean that there is chirality. In some cases, there is no optical activity.
In particular, if there are two or more chiral carbon atoms, there are cases where the material has no optical activity. This is called a meso compound.
When does a compound become one with no optical isomer in spite of the presence of a chiral center? For example, for 2,3-dibromobutane, four structural formulas can be written as follows.
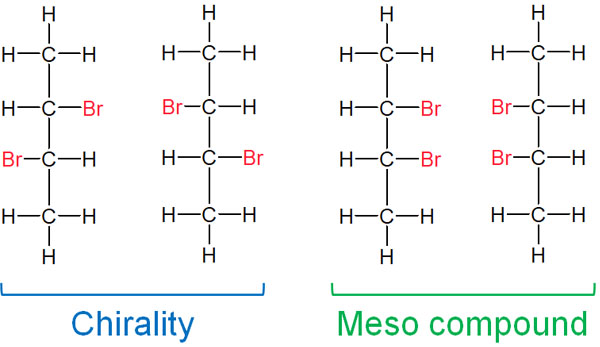
As noted in the Fischer projection, the two compounds on the left have chirality. On the other hand, the two compounds on the right do not have chirality. In the meso compounds, if you rotate them by 180°, they become exactly the same compound. Even if there is a chiral center, if it is symmetrical, it is not an optical isomer.
Because two substances can be superimposed, meso compounds can be said to have achiral properties. A compound with no optical activity is a meso compound.
Understanding Three-Dimensional Placement in RS Notation System
DL notation is sometimes used to describe an optically active molecule. However, DL notation is a relative method. Therefore, in organic chemistry, it is common to use RS notation, which is an absolute notation, instead of DL notation.
For the RS notation method, follow the rules below.
- Rank the compounds in order of their atomic number.
- If the substituents are the same, judge by the substituents attached to the atoms.
For example, with the following molecule, the ranking would be as follows.
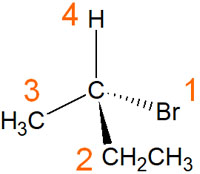
The highest atomic number in this molecule is the bromine atom. Also, the lowest ranking is the hydrogen atom.
The second-largest atomic number is the carbon atom. However, since the same carbon atoms are bound to each other, in this case, it is determined by the substituent group attached to the carbon atom. Therefore, between the methyl group (-CH3) and the ethyl group (-CH2CH3), the ethyl group has a higher priority.
In this way, the RS notation gives a ranking for each substituent.
Clockwise is an R, and counterclockwise is an S
After ranking, you need to decide whether it’s an R or an S. In RS notation, the following steps will help you decide.
- Put the fourth substituent at the back.
- Clockwise is an R; counterclockwise is an S.
For example, in the compound we just mentioned, the fourth substituent is a hydrogen atom. Because we need to put the hydrogen atom at the back, we have to look at the compound from the bottom of the molecule.
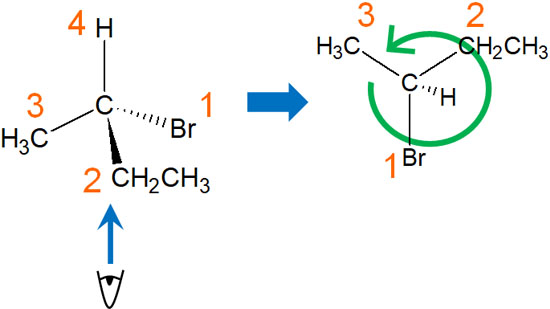
You need to imagine the three dimensions of the molecule in your mind. Or you can write it down on a piece of paper. Then you will be able to determine whether the ranking of the substituents is clockwise or counterclockwise. In the case of this molecule, it’s counterclockwise. Therefore, it is determined to be an S.
For reference, the EZ isomer in cis-trans is also ranked according to the atomic number to determine whether it is E or Z. As with EZ-isomers, the RS of chiral compounds is ranked using the same concept.
Two Chiral Carbon Atoms Produce Diastereomers
So far, we have explained the properties of compounds with chiral centers and RS notation. So what happens when there are two or more chiral carbon atoms in a molecule?
We have already discussed meso compounds. However, there are very few compounds that are symmetrical meso compounds, and most of them have chirality. So we have to understand the concept of diastereomers as well as enantiomers.
With two chiral centers, there are four patterns of isomers, as follows.
- R, R
- R, S
- S, R
- S, S
Among these compounds, diastereomers are those that are stereoisomers but not enantiomers. As an example, the relationship between enantiomers and diastereomers in the following compounds is noted.
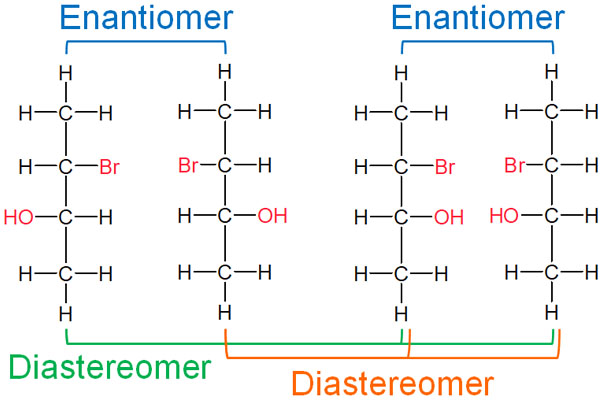
The difference between enantiomers and diastereomers is whether or not they are in a mirror image relationship. If they have chirality but are not enantiomers, they are diastereomers.
Enantiomers have the same physical properties, such as melting point and boiling point. Their chemical properties are also the same except for their optical rotation.
Diastereomers, on the other hand, have different physical properties. Separation of enantiomers requires advanced technology. Diastereomers, on the other hand, are easier to distinguish than enantiomers because of their different physical properties.
Thinking About Optically Active Compounds in Stereochemistry
In organic chemistry, in many cases, molecules are described in planar form. However, in the real world, molecules exist in three dimensions. Therefore, we have to think in terms of stereochemistry.
One of the important areas of stereochemistry is optical isomers. By having a chiral center, they become optically active. This results in enantiomers. With the exception of their optical rotation and the bioactivity of pharmaceuticals, the properties of enantiomers are the same.
Therefore, although it is difficult to distinguish between them, they are not the same compound and must be clearly identified. So, use RS notation to distinguish the difference between the two.
Also, if there are two or more chiral carbon atoms, they will give rise to diastereomers as well as enantiomers. In some cases, when two or more chiral centers are present, they exist as meso compounds and have no optical activity.
By understanding the nature of these optical isomers, our knowledge of stereochemistry can be deepened. It is difficult because you need to think of molecules in three dimensions, but you should be able to distinguish between these compounds.