There are a great many molecules in organic compounds that have double bonds. When these molecules react with other reagents, addition reactions occur. When addition reactions occur, the double bond of the alkene changes to a single bond, and the product is obtained.
In organic chemistry, the addition reaction of alkenes is the basis of the reaction. However, there are several types of addition reactions of alkenes, each with a different reaction mechanism. Therefore, it is necessary to distinguish how organic chemical reactions proceed.
In addition reaction of alkenes, we have to learn several reactions, such as Markovnikov’s rule, anti-Markovnikov’s rule, hydroboration, and halogen addition.
If we check the reasons why organic reactions occur, we will be able to understand why and how alkene addition reactions occur. In this section, we will review the mechanism of addition reactions to double bonds.
Table of Contents
Electrophilic Addition Reaction Caused by Double Bonds
One of the organic chemical reactions that occur for alkenes (double bonds) and alkynes (triple bonds) is an addition reaction.
In organic chemistry, there are several reaction mechanisms. Among them, addition reactions are organic chemical reactions in which all the reacting compounds are contained in the molecule of the product compound. The following synthetic reactions are examples of addition reactions.
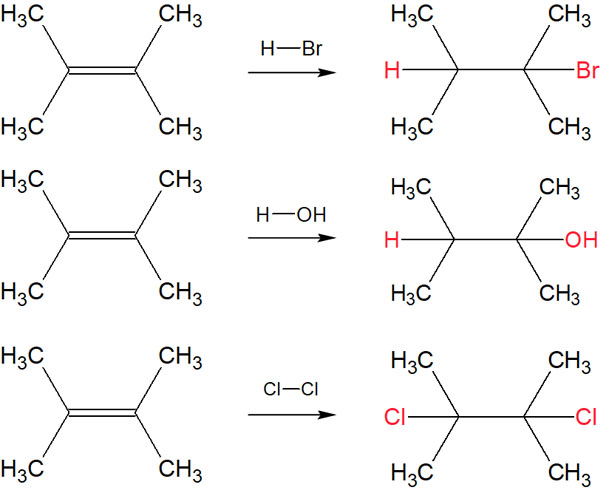
These are all addition reactions. All the reacted compounds are present in a single molecule. That is why they are called addition reactions.
Addition reactions are also known as electrophilic addition reactions. Double and triple bonds have more electrons than single bonds. They are electron-rich and provide electrons to an electron-deficient reagent (electrophilic reagent) to cause a chemical reaction.
Reaction Mechanism of Addition Reactions to Alkenes with HX (HBr)
What is the reaction mechanism for these addition reactions to alkenes? Since the HX (hydrogen halide) addition reaction is the simplest of the electrophilic addition reactions to alkenes, it is common to explain this reaction mechanism first.
X stands for halogen. Therefore, understand that HX is HCl (hydrogen chloride) or HBr (hydrogen bromide). HBr is frequently used as an example, especially in addition reactions to alkenes.
Double and triple bonds are rich in electrons, as mentioned earlier. Double bonds are formed by π bonds (π-electrons); π bonds are weak bonds and can give electrons to other molecules.
Halogens, on the other hand, are known as atoms with strong electronegativity. Therefore, when a hydrogen atom is attached to a halogen, the molecule becomes highly polarized. HCl (hydrogen chloride) and HBr (hydrogen bromide) have both positive and negative charges in the molecule.
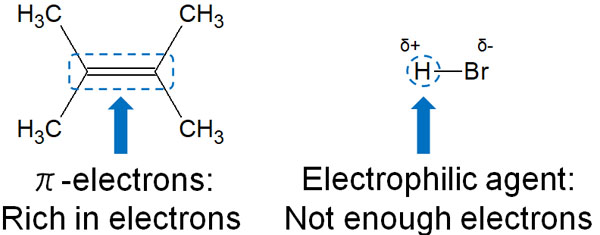
The hydrogen atoms in HCl and HBr are electron-deficient because electrons are attracted to the halogen, which means that they are strongly seeking electrons. Since HCl and HBr are deficient in electrons, they can be considered electrophiles.
These electrophiles and alkenes react chemically with each other. Specifically, the π-electrons forming the double bond attack the hydrogen atoms (electron-deficient atoms). Then, a proton (hydrogen) is added to the alkene. As a result, a carbocation is produced as an intermediate.
For example, when an alkene reacts with hydrogen bromide, the result is as follows.
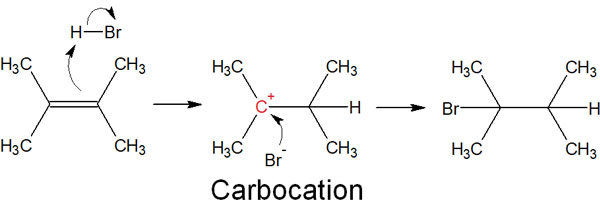
Then, the bromide ion that was just produced attacks the carbocation. The bromide ion produced is negatively charged and also has unshared electron pairs (lone pairs). In other words, it has a lot of electrons.
In contrast, the carbocation is positively charged and requires electrons. Therefore, the bromide ion attacks and adds to the carbocation. Thus, an alkyl halide compound is formed.
Markovnikov’s Rule (Markovnikov Addition): Stability of Carbocation
The addition reaction of HX to alkenes proceeds in this way. In order to make it easier to understand, we used symmetrical alkene in the previous explanation. However, in the case of an asymmetric alkene, multiple compounds seem to be generated.
For example, in the following case, which compound will be produced?
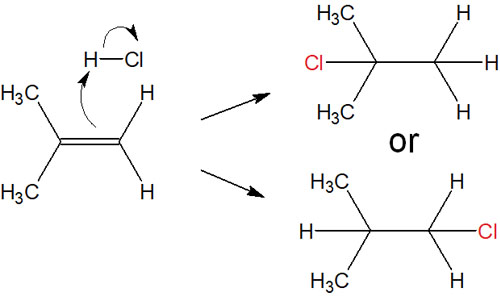
For this problem, there is a rule for compounds produced by electrophilic addition reactions to alkenes. In the addition reaction, the hydrogen is added to the carbon atom with fewer alkyl groups. This rule of thumb is called Markovnikov’s rule (Markovnikov addition).
Why does Markovnikov’s rule occur? The reason for this is the carbocation of the intermediate. The carbocations have an order of stability, as shown below.
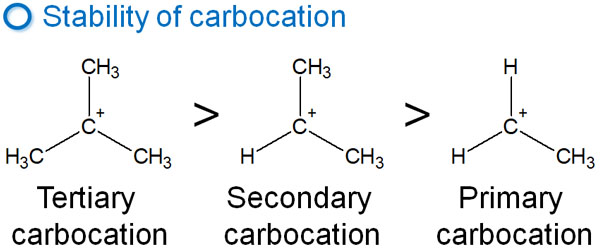
Therefore, when the intermediate carbocation is formed, the tertiary carbocation is preferentially formed. If the tertiary carbocation is not formed, the secondary carbocation is formed.
Checking the previous addition reaction, the intermediate is shown below.
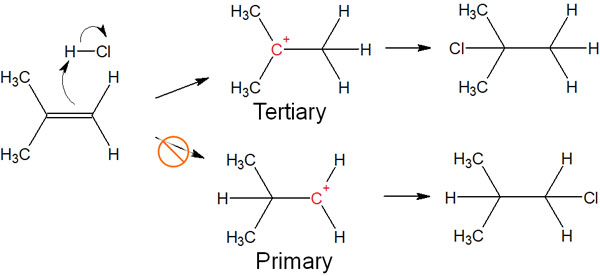
If we check the intermediates of carbocation, one produces tertiary carbocation, and the other produces primary carbocation.
In terms of carbocation stability, the tertiary carbocation is the most stable carbocation. Therefore, tertiary carbocations are preferentially formed instead of primary carbocations. As a result, the addition reaction to alkenes proceeds according to the Markovnikov addition.
-A Rule Different from the Saytzeff Rule
There are several rules in organic chemistry. One of them is Markovnikov’s rule. Markovnikov’s rule is a law that should be considered in alkene addition reactions.
However, some people confuse Markovnikov’s rule with the Saytzeff rule. The Saytzeff rule is a rule that should be taken into account for elimination reactions. The synthetic reaction to make an alkene (double bond) from an alkane is an elimination reaction. It is important to understand that the Markovnikov addition and the Saytzeff rule are completely different rules.
Proton Transfer in the Carbocation Rearrangement
Understanding the stability of the carbocation also helps us to understand other organic chemical reactions. We can understand why carbocation rearrangement occurs in addition reactions as well as Markovnikov’s rule.
When an addition reaction occurs for a double bond, a proton (hydrogen atom) can be transferred to a neighboring carbon atom. This is called a carbocation rearrangement. As a result of the proton transfer, different compounds can be produced.
An example is the following organic chemical reaction.
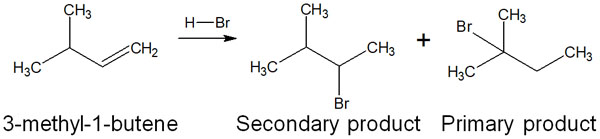
When an addition reaction occurs according to Markovnikov’s rule, it would seem that only the secondary product shown in the above figure would be produced. In reality, however, a different compound is produced. Why does this kind of organic chemical reaction occur? The reason for this is the carbocation rearrangement.
The carbocation wants to be in a more stable structure. Therefore, after the carbocation intermediate is formed, a proton (hydrogen atom) is transferred to the neighboring carbon. In this synthetic reaction, the secondary carbocation becomes a tertiary carbocation by carbocation rearrangement.
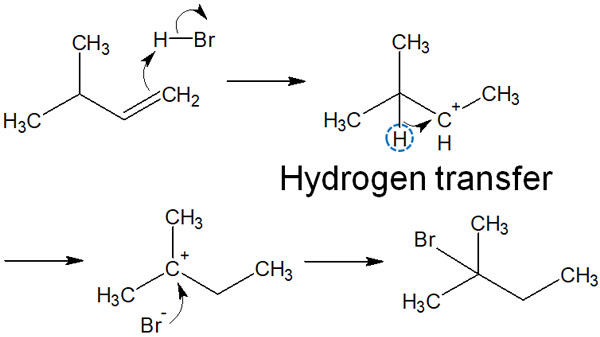
The main product can then be obtained by the attack of the halogen on the tertiary carbocation.
If a more stable carbocation is produced, a carbocation rearrangement occurs. Carbocation rearrangements also only involve the transfer of a neighboring hydrogen atom. It is important to understand that protons (hydrogen atoms) do not move from every part of the molecule.
Addition of Water to Alkenes (Hydration)
After learning the reaction mechanism with HX, you will be able to understand the addition reaction of water. Water can be thought of as a type of reagent, and the addition of water to alkenes occurs.
However, water is a weak acid and cannot produce carbocation. In other words, the addition of water (hydration) to alkenes does not occur. Therefore, by adding sulfuric acid (H2SO4) as an acid catalyst, the water addition reaction can proceed.
In the presence of an acid catalyst (e.g., sulfuric acid), oxonium ions (H3O+) are generated. Oxonium ions are strong acids, so they can give H+ to alkenes.
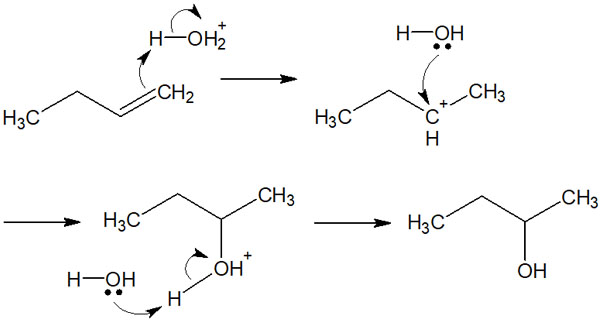
The addition reaction then proceeds according to Markovnikov’s rule of regioselectivity. Understand that the addition reaction of water is the same as the addition reaction of HX, except that an acid catalyst is required.
Since it is a strong acid-catalyzed chemical reaction, there is no OH– in the aqueous solution. Therefore, in this hydration reaction, only oxonium ions (H3O+) and water molecules are involved in the chemical reaction.
Reaction Mechanism of Addition Reaction by Alcohol
If we understand what we have learned so far, we can understand that the reaction mechanism is almost the same for both the addition reactions of HX and water. In the case of water, an acid catalyst is required.
Once we understand this fact, we can understand the reaction mechanism of the addition reaction with alcohol. We have just explained the addition reaction with water, which is hydration. If alcohol such as methanol or ethanol is used as a solvent instead of water, the addition reaction with the alcohol will proceed.
Like the water addition reaction, the addition reaction with alcohol uses an acid catalyst. Then, H+ is bonded to the alcohol by the reaction of the alcohol and the acid catalyst.
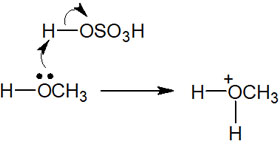
Thereafter, the alcohol addition reaction proceeds by exactly the same reaction mechanism as the water addition reaction. The result is as follows.
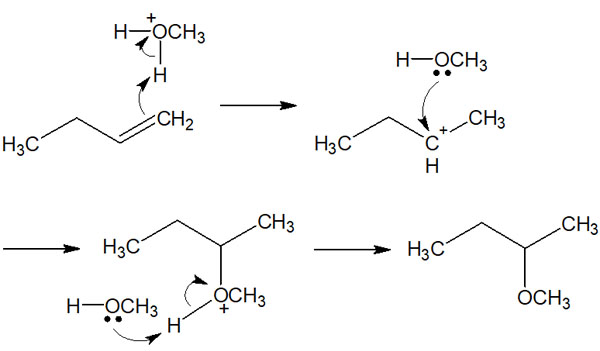
Once you understand the addition reactions to alkenes, you will be able to synthesize many compounds. By changing the reagent to be reacted with, you can change the product obtained.
Hydroboration-Oxidation Reaction: Anti-Markovnikov Rule
We have discussed chemical reactions that follow the Markovnikov addition. In all of these synthetic reactions using water and alcohols, Markovnikov’s rule takes precedence, and therefore, multi-substituted alkanes are synthesized. However, is it possible to synthesize alkanes with fewer substituents?
There is an addition reaction that does not follow the Markovnikov addition, namely the anti-Markovnikov rule. There are several types of anti-Markovnikov rule, and a typical organic chemical reaction is the synthesis of alcohols by hydroboration.
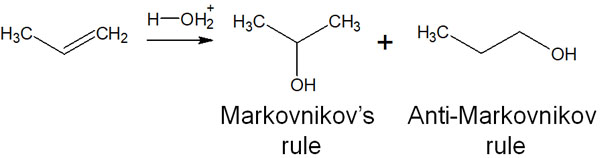
What kind of chemical reaction is hydroboration, which is an anti-Markovnikov rule? Hydroboration refers to the addition of borane (BH3) to an alkene.
Borane is known to be a Lewis acid. Boranes have an unoccupied orbital and can accept electrons. Borane is a Lewis acid and is a powerful electrophilic reagent that accepts electrons present in the double bond of alkenes.
However, when borane is added to an alkene, an opposite addition reaction to the Markovnikov addition occurs. It looks like the following.

When the borane undergoes an addition reaction, the intermediate carbocation is not produced. Therefore, the stability of the carbocation is irrelevant, and the reaction becomes the anti-Markovnikov rule.
Why does the anti-Markovnikov rule apply to the transition state? The reason is related to the steric hindrance. A carbon atom with fewer substituents has less steric hindrance. Therefore, boron atoms bond with carbon atoms that have fewer substituents.
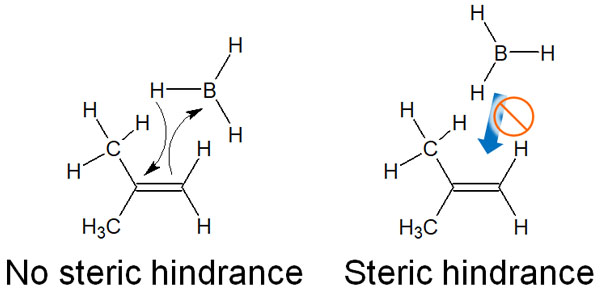
Since the addition reaction takes place under conditions of low steric hindrance, the hydroboration occurs with an anti-Markovnikov rule. Since no carbocation is produced, the stability of the carbocation is not relevant, as mentioned above. Naturally, the carbocation rearrangement does not occur. Due to steric hindrance, an anti-Markovnikov rule occurs.
After the addition of a borane by the anti-Markovnikov rule, hydrogen peroxide (H2O2) is used to oxidize the alkylborane.
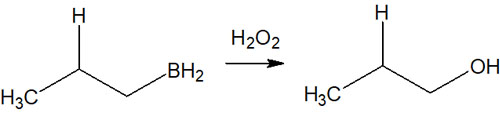
Hydroboration is frequently used in alcohol synthesis. Alkylboranes are synthesized by hydroboration and then oxidized by hydrogen peroxide, which converts the alkylboranes to hydroxy groups (-OH). Thus, alcohol synthesis is possible.
Anti-Addition Reactions with Halogens
After understanding what we’ve discussed so far, we will explain the addition reaction by halogens. Why is the addition reaction of alkenes with halogens so important? It is because stereochemistry is involved. Although we have not considered stereochemistry in our previous explanations, let’s consider stereochemistry using halogens as an example.
When halogens such as Cl2 and Br2 undergo addition reactions, they are known to cause anti-additions. If the addition occurs on the same side, it is called syn-addition. On the other hand, when the addition reaction occurs on the opposite side, it is called anti-addition.
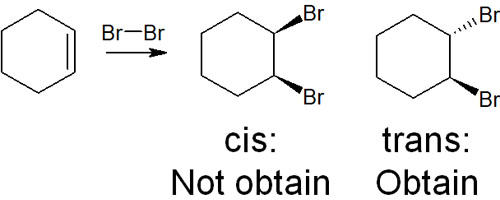
Instead of syn-addition, halogens always undergo anti-addition. Why does it cause an anti-addition?
When a halogen is added to a double bond, the reaction proceeds without producing a carbocation. Like hydroboration, the reaction proceeds at the same time. The reaction of halogens also produces a cyclic ionic intermediate. For example, when bromine reacts with a double bond, a cyclic bromonium ion is produced as an intermediate.
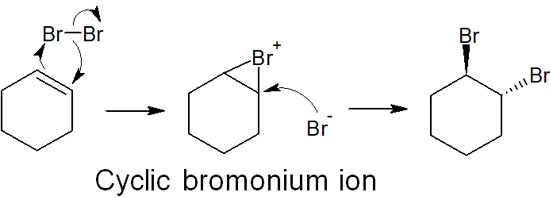
The bromide ion then attacks from the other side. This allows the synthetic reaction to proceed by anti-addition rather than syn-addition. Since the halogen ion attacks stereoselectively, we must take stereochemistry into account.
As mentioned above, no intermediates of the carbocation are formed. Therefore, the addition of halogens does not cause a carbocation rearrangement.
-When a Chiral Center Is Created, Stereoselectivity Is Important
It is important in stereochemistry that the addition reaction by halogen is anti-addition. When a chiral center is formed as an optical isomer, we have to consider what kind of shape the molecule will be.
If a carbocation intermediate is formed, the compound with the chiral center will be racemic mixtures. This is because after the carbocation is formed, the compound (nucleophile) attacks it from above or below. Because of racemization, the two optical compounds will mix together.
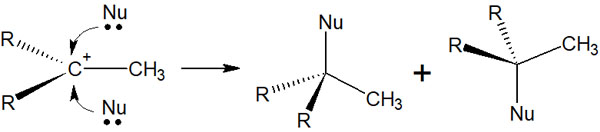
On the other hand, in the addition reaction with halogens, as mentioned above, only anti-addition occurs. Therefore, no racemization occurs and only a specific compound can be obtained, even if there is a chiral center.
Water or Alcohol Is Used as a Solvent to Synthesize Trans Compounds
The application of halogen reactions allows us to synthesize alcohols and alkoxy compounds. Anti-addition gives trans compounds.
In these syntheses, water or alcohols (methanol, ethanol, etc.) are used as solvents, for example. In this way, the water or alcohol, rather than the bromine ion, will attack the intermediate to give the alcohol.
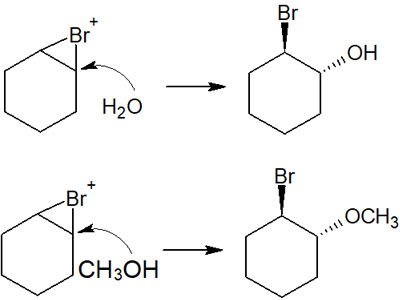
If water is used as the solvent, the substituent will be a hydroxy group (-OH). On the other hand, if methanol (CH3OH) is used as the solvent, the methoxy group (-OCH3) will be added. Depending on the solvent used, the compound you want to synthesize can be changed.
Why does water or methanol attack instead of bromine ion? This is because water or methanol is used as the solvent.
As a solvent, there is a very large amount of water (or alcohol) in the surroundings of the compound. So it is more likely that the solvent will nucleophilic attack rather than the low concentration of bromine ions. This is why we are able to obtain these compounds.
Hydroboration and Catalytic Hydrogenation Are Syn-Addition
In addition, when we consider stereochemistry, the synthesis of alcohols by hydroboration and oxidation is understood to be syn-addition. When a borane is used in the synthesis, it causes syn-addition without a carbocation intermediate. Subsequently, the alcohol is obtained by oxidation.
Another addition reaction, catalytic reduction with hydrogen, is known to be a syn-addition.
In catalytic reduction, the air is filled with hydrogen, and carbon palladium is added to the solution to cause the reduction reaction. The hydrogen is adsorbed on the palladium carbon, and at this time, syn-addition occurs.
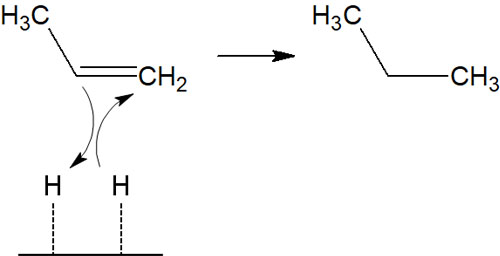
Since only syn-addition proceeds, racemization does not occur even in the presence of a chiral center. Also, the hydrogen addition reaction proceeds without the carbocation intermediate.
In the absence of a chiral center, there is no need to take into account the difference between the syn-addition and anti-addition reactions. However, if a chiral center is formed after the reaction, stereochemistry is important.
Learn Why Addition Reactions of Alkenes Occur and Regioselectivity
In high school chemistry, you only need to remember the compounds that are produced after an organic chemical reaction occurs. However, in college organic chemistry, you have to understand the reaction mechanism. In such cases, the addition reaction of alkenes is one of the first steps in organic chemistry.
Even though they are basic, there are various types of addition reactions in organic chemistry. There are many organic chemists who use addition reactions when performing synthetic reactions. This is because many compounds can be synthesized by changing the reagents.
There are many types of reactions. In addition, you need to understand Markovnikov’s rule and the difference between syn-addition and anti-addition. Furthermore, you should learn even regioselectivity. However, the reaction mechanisms are all similar.
Try to understand how each addition reaction proceeds.