The SN1 and SN2 reactions are two methods that are frequently used in synthetic reactions in organic chemistry. They are convenient synthetic reactions that all organic chemists are sure to use them.
The SN1 and SN2 reactions are synthetic reactions, called nucleophilic substitution reactions, in which the substituents are swapped.
However, the reaction mechanism differs depending on the type of compound to be reacted with. The easiness of the synthetic reaction to proceed is also different. Without learning these reaction mechanisms, you will not be able to obtain your desired compounds. The solvent used in the synthesis is also important.
In this section, we will explain the basic organic chemical reactions, SN1 and SN2 reactions, and how the synthetic reactions proceed according to the reaction mechanisms.
Table of Contents
- 1 Nucleophile (Nu) Is a Substance with High Electron Density
- 2 The SN1 Reaction Is a Two-Step Reaction
- 3 Three-Dimensional Inversion SN2 Reaction: Walden Inversion
- 4 Comparing the Difference Between the SN1 and SN2 Reactions
- 5 Differences in Stability Due to Solvent Effects
- 6 Distinguishing the Ease of Nucleophilic Substitution Reactions Occur
Nucleophile (Nu) Is a Substance with High Electron Density
Before learning about nucleophilic substitution reactions, we must understand what a nucleophile (Nu) is in the first place. In a nucleophile (or nucleophilic reagent), they all have a high electron density. As a result, they try to make new bonds by attacking other compounds.
So, when does a high electron density increase and act as a nucleophile? If the following conditions are met, it is likely to become a nucleophile.
- It has unshared electron pairs (lone pairs).
- High electronegativity.
Atoms with high electronegativity tend to attract many electrons and thus become electron-rich. In addition, the presence of unshared electron pairs makes it easier for lone electron pairs, which are not involved in bonding, to attack other molecules.
- OH–
- NH3
These are widely known to function as nucleophiles. In addition, they tend to become nucleophilic reagents when they are negatively charged, such as anions.
Nucleophilic Substitution Reaction with the Presence of a Leaving Group (L)
Does the presence of a nucleophilic agent always cause a nucleophilic substitution reaction? Of course not. In addition to the presence of a nucleophile, there is another condition for a nucleophilic substitution reaction: the presence of a leaving group (L). The presence of the leaving group allows the nucleophilic substitution reaction to proceed.
Molecules (or atoms) that are easily stabilized by receiving electrons tend to be the leaving groups. Typical leaving groups are halogens.
- Chlorine (Cl)
- Bromine (Br)
- Iodine (I)
These tend to be the leaving groups (L). With the presence of leaving groups, the reaction proceeds as follows, for example.
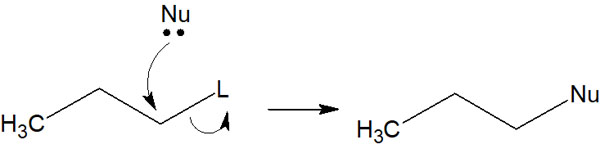
It’s hard to understand with symbols like Nu and L. In an actual synthetic reaction, it looks like the following.
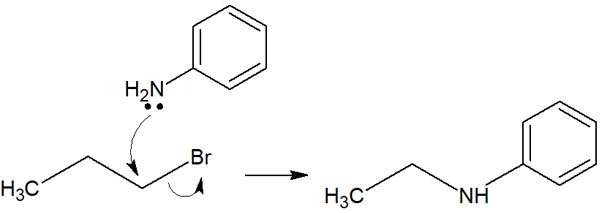
In this way, the leaving group and the nucleophilic reagent are replaced. This is why it is called a substitution reaction. Although halogens can function as nucleophiles, they are frequently used as leaving groups.
Although the reaction mechanisms are different, both SN1 and SN2 reactions involve leaving groups.
The SN1 Reaction Is a Two-Step Reaction
Let’s check how the synthetic reaction actually works.
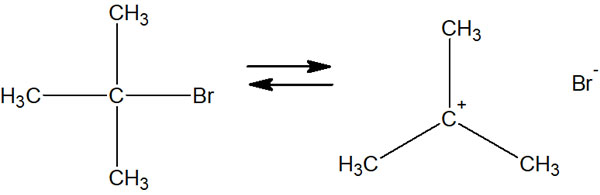
In the SN1 reaction, the first step of the synthetic reaction is the departure of the leaving group. This results in the formation of a carbocation. All SN1 reactions start with the leaving group separating.
You may be wondering why the bond breaks and leaves on its own, even though it is strongly bonded by a covalent bond. However, the leaving group will try to break the covalent bond and leave on its own.
For example, halogens are known for their strong electronegativity. As a result, they are polarized into positive and negative charges in the molecule. Because of the high polarization of the charge, the halogen wants to leave from molecule whenever it gets a chance. So, the leaving group leaves the molecule, and a carbocation is formed.
But the carbocation is an unstable intermediate, so the compound tries to maintain its molecular shape by re-capturing the separated halogen.
On the other hand, what if there is a nucleophile in the same solution? In this case, the nucleophilic reagent attacks the carbocation at the moment the leaving group leaves the molecule.
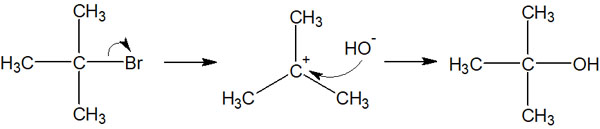
The nucleophile attacks the carbocation faster than the carbocation tries to catch a leaving group (such as a halogen). As a result, the SN1 reaction occurs.
- The leaving group leaves and gives rise to a carbocation.
- The nucleophilic agent attacks the carbocation.
Because of the two reactions involved, the SN1 reaction is said to be a two-step reaction.
SN1 Reaction Occurs When the Carbocation Is Stable
The formation of the carbocation is a prerequisite for the SN1 reaction. Therefore, how easily the carbocation is formed plays a major role in the reaction rate. To be more precise, the more stable the carbocation of the intermediate is, the faster the reaction rate.
There is no doubt that the carbocation is an unstable intermediate. However, if the intermediate is not formed, the SN1 reaction will not occur due to the leaving group leaving. Therefore, the stability of the carbocation is very important as a factor in whether or not the SN1 reaction occurs.
The carbocation is stable in the following order.
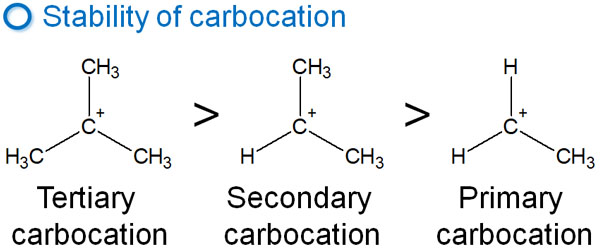
The more alkyl chains that are attached to the carbocation, the more stable the intermediate is likely to be. In fact, it does not naturally give rise to a primary carbocation. On the other hand, a tertiary carbocation tends to be a stable structure.
The Rate of the SN1 Reaction Depends on a Single Molecule
Why is it called the SN1 reaction? This is because there is only one molecule in a nucleophilic substitution reaction that is involved in the reaction rate (rate-determining step). The rate-determining step (or rate-limiting step) is the factor that determines the reaction rate. Since there is only one rate-determining step, we call it the “SN1 reaction” using the number 1.
What is the rate-determining step in the SN1 reaction? It is not the nucleophile that determines the reaction rate in the SN1. Rather, the rate-limiting step is the formation of the carbocation.
As soon as the carbocation is produced, the nucleophilic reagent attacks the carbocation. However, if no carbocation is produced, the SN1 reaction does not occur. No matter how highly nucleophilic the reagent (high electron density reagent) is, the reaction rate of the SN1 reaction does not change rate, because the important thing is whether or not the carbocation is produced.
In the SN1 reaction, only the compound with the leaving group attached is involved in the reaction rate. Nucleophiles are not involved in the reaction rate.
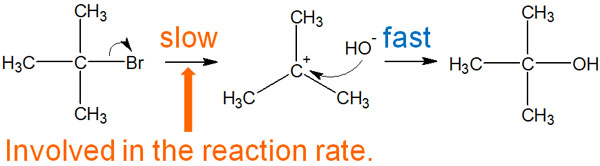
The rate-determining step of the SN1 reaction may be illustrated using an energy diagram. The energy diagram is shown below.
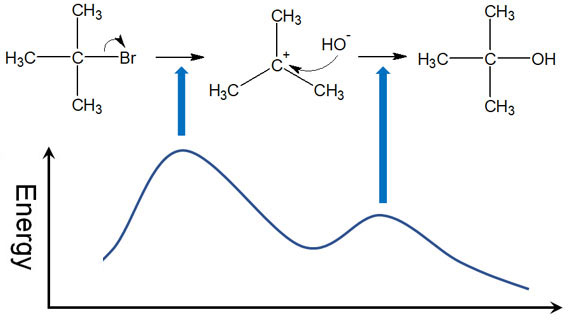
The SN1 reaction requires the highest energy at the beginning when the carbocation is formed. The high energy state is called the transition state. A large amount of energy is required to reach the transition state.
On the other hand, when the nucleophile is attacking, the energy of the transition state is low. Therefore, once the initial reaction is underway, the rest of the chemical reaction proceeds automatically. For this reason, the first step of the SN1 reaction is the rate-limiting step.
The ease of the SN1 reaction occurring is as follows.
- Tertiary > Secondary > > > Primary > Methyl cation
We have already mentioned that the SN1 reaction does not occur with primary carbocations. This is because primary carbocations are almost never formed. On the other hand, the SN1 reaction proceeds at a slower rate with secondary carbocations. The reaction rate is faster in the case of tertiary carbocations.
Note that even with primary carbocations, allyl cations and benzyl cations exceptionally proceed with the SN1 reaction.
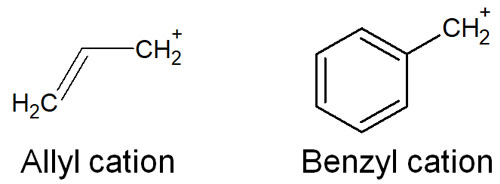
In an allyl and benzyl cation, there is a double bond next to it. This allows resonance structures to be written and stabilizes the structure of the cation. As a result, the SN1 reaction occurs.
In Stereochemistry, the SN1 Reaction Results in Racemic Mixture
In the stereochemistry of the SN1 reaction, racemization occurs. In other words, if we have to consider stereochemistry, we should understand that two substances are produced by the racemate.
After the carbocation is formed, the nucleophile can attack the chiral carbon atom from two directions, from above or below. The result is racemization.
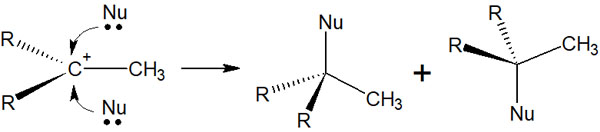
Of course, if the substituents attached to the carbocation are the same, the stereochemistry is irrelevant because it is not a chiral carbon atom. Therefore, the racemic mixture is not formed. However, if the substituents attached to the chiral carbon are all different, a mirror image isomer will be produced.
Racemic isomers are important in stereochemistry. Understand that in the SN1 reaction, racemization causes the two products to mix together.
Three-Dimensional Inversion SN2 Reaction: Walden Inversion
On the other hand, what does the SN2 reaction look like? Unlike the SN1 reaction, which produces a racemate, in the SN2 reaction, the compound is sterically inverted by an attack from the opposite side.
The SN2 reaction does not produce a carbocation. Instead, the nucleophile attacks from the opposite side, producing a sterically inverted compound after the transition state, as shown below.
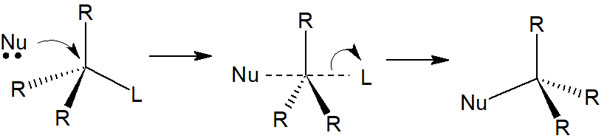
Thus, the nucleophile attacks in such a way that it replaces the leaving group.
When a carbocation is produced, as in the SN1 reaction, the nucleophile can attack from two directions: above and below. On the other hand, in the SN2 reaction, the nucleophile only attacks from the opposite side, and racemization does not occur. In this case, only one sterically inverted compound is produced.
The reaction in which the chiral center of the molecule changes is called Walden inversion. There are several organic chemical reactions that cause Walden inversion, and the SN2 reaction is one of them.
Tertiary Alkyl Groups Don’t Cause Reactions Due to Steric Hindrance
The SN2 reaction is more likely to occur with methyl and primary alkyl groups. The reaction rate is slower with secondary alkyl groups, and the SN2 reaction does not occur with tertiary alkyl groups.
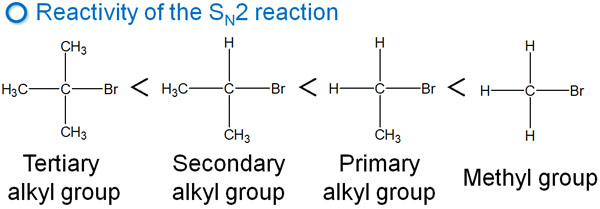
In other words, consider that the order of reactivity is the reverse of the SN1 reaction. Why does the SN2 reaction not occur with tertiary alkyl groups? Steric hindrance is involved in this.
In tertiary alkyl groups, many substituents are attached to them. Even if the nucleophile tries to attack from the opposite side, the substituents prevent the nucleophile from approaching the target carbon atom. As a result, the SN2 reaction cannot occur.
For example, suppose that a nucleophilic substitution reaction is carried out for tert-butyl bromide. In this case, the SN2 reaction does not occur due to a steric hindrance. On the other hand, the SN2 reaction will occur with bromoethane.
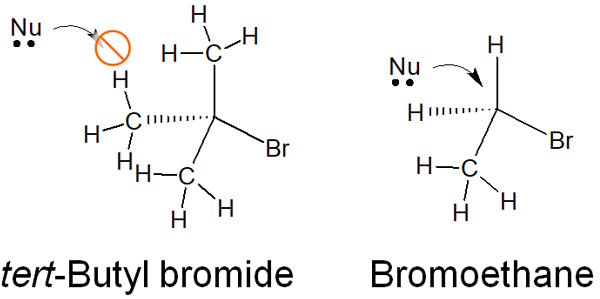
If we draw a structural formula like this, we can understand why a nucleophile cannot attack from the opposite side in a tertiary alkyl group. In organic chemistry, it is necessary to consider steric hindrance.
Note that although tert-butyl bromide does not cause the SN2 reaction, it causes the SN1 reaction. This has been explained earlier.
-Cyclic Compounds Such as Cyclohexane Have Lower Reactivity
For reference, it is known that the reactivity of the SN2 reaction is slower in cyclic compounds such as cyclohexane than in other compounds. What is the reason for this?
In cyclohexane, it is known that there is a steric hindrance caused by hydrogen atoms. This is called the 1,3-diaxial interaction—the hydrogen atoms in the 1 and 3 positions of cyclohexane cause steric hindrance in the axial.
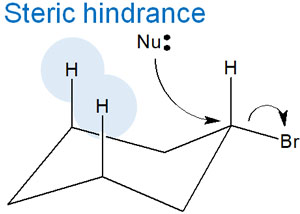
In organic chemistry, differences in reaction rates can often be explained by steric hindrances.
Reactivity Varies Depending on the Type and Concentration of Nucleophilic Reagents and Leaving Groups
In the case of the SN2 reaction, the nucleophile is involved in the rate of the reaction; in the SN1 reaction, the nucleophile was not involved in the rate-limiting step. In the SN2 reaction, the nucleophile attacks from the opposite side. This is why it is involved in the rate of the reaction.
The stronger the nucleophilicity (basicity) and the higher the concentration of the nucleophile, the more likely the SN2 reaction will occur. Also, two factors are involved in the rate of the SN2 reaction. For this reason, the number 2 is used in the SN2 reaction.
In addition, even with the same nucleophilic reagent, the more negatively charged a compound is, the stronger its nucleophilic properties will be. As mentioned above, the stronger the basicity, the stronger the nucleophilic property. The order of nucleophilicity is as follows.
- CH3O– > CH3OH
- C6H5NH2 > C6H5NH3+
This is easy to understand because it is natural that the basicity becomes stronger with a negative charge. The energy diagram of the SN2 reaction is as follows.
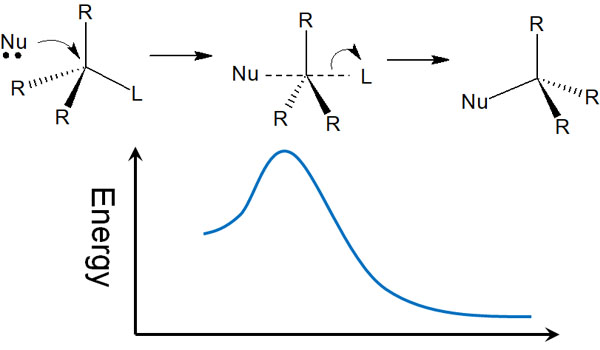
When energy is added, a transition state is created. In this case, both the nucleophile and the reactive compound are involved in the reactivity.
Williamson Ether Synthesis Is an SN2 Reaction with Halogen Substitution
The synthetic reactions by SN2 reactions are also used in name reactions. In organic synthesis, there are many name reactions that utilize the name of the discoverer. One of these name reactions is the Williamson ether synthesis.
It is important to understand that Williamson ether synthesis is, in essence, a method of synthesizing ethers through the SN2 reaction. Therefore, the content is very simple if you understand what we have been talking about. The oxygen atom of the alcohol attacks the carbon atom that the halogen is bonded to, and the ether is synthesized by the SN2 reaction.
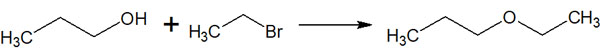
When performing ether synthesis, Williamson ether synthesis is frequently used.
The SN2 reaction is beneficial in all organic reactions, not just Williamson ether synthesis. In addition to alcohols, by using molecules with other functional groups, many types of molecules can be made. It is as follows.
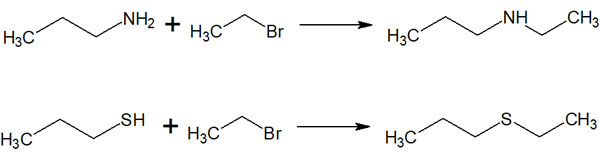
Nucleophilic substitution reactions can be used to synthesize a very large number of compounds. All organic chemists use nucleophilic substitution reactions.
Comparing the Difference Between the SN1 and SN2 Reactions
Let’s try to sort out what the differences are when comparing SN1 and SN2 reactions. They can be summarized as follows.
| SN1 reaction | SN2 reaction | |
| Reaction rate | Reaction compounds | Nucleophiles and reaction compounds |
| Reaction | Single-molecule reactions | Double-molecule reactions |
| Number of reactions | Two-step reaction | One-step reaction |
| Products | Racemic mixture | Stereoscopic inversion |
Also, depending on the alkyl chain attached, such as tertiary or secondary, whether the reaction is an SN1 reaction or an SN2 reaction will differ. It is as follows.
| Tertiary | Secondary | Primary | Methyl | |
| SN1 | Excellent | Good | No reaction | No reaction |
| SN2 | No reaction | Good | Excellent | Excellent |
Depending on the substituents in the reaction compound, which reaction takes place will vary.
Leaving Groups (the Nature of the Halogen) Are Involved in the Reactivity
One thing that SN1 and SN2 reactions have in common is that the leaving group is involved in the reactivity. The higher the reactivity of the leaving group attached to the compound, the easier it is for the organic synthesis reaction to occur.
What kind of leaving group is highly reactive? The more stable the leaving group is after separation, the higher the leaving ability. The more stable the compound (conjugate base) is after leaving, the more the leaving group wants to leave and move freely, and thus the higher the leaving ability.
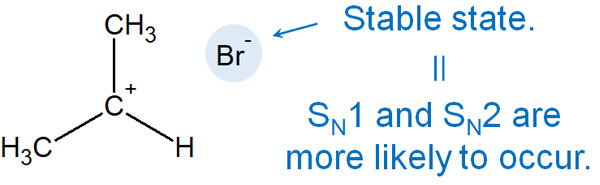
Specifically, what kind of molecule has a higher leaving capacity? In this regard, the higher the acidity (lower the basicity) of the substance produced by the leaving, the better the leaving capacity.
The stronger the acidity of a substance, the more likely it is to exist as an ion in solution. Therefore, the more acidic the compound is, the more stable it will be after leaving. The acidity of halogens, in order, is as follows.
- HI (hydrogen iodide) > HBr (hydrogen bromide) > HCl (hydrogen chloride) > HF (hydrogen fluoride)
Therefore, the leaving capacity in the nucleophilic substitution reaction is in the following order.
- I (iodine) > Br (bromine) > Cl (chlorine) > F (fluorine).
When comparing iodine and fluorine, fluorine has a higher degree of electronegativity. Therefore, fluorine has a greater degree of polarization and seems to be the better leaving group. However, considering the stability after leaving, iodine is superior because of its higher acidity.
Differences in Stability Due to Solvent Effects
In the nucleophilic substitution reaction, the reactivity varies depending on the solvent used. This is called the solvent effect. The solvent effect differs between the SN1 and SN2 reactions; it is necessary to select the right solvent to use.
The three main solvents used in organic synthesis are as follows.
- Protic solvent
- Aprotic solvent
- Non-polar solvent
Let’s check what each solvent is.
-Polar Protic Solvents Strongly Stabilize Ions
Methanol (CH3OH), ethanol (CH3CH2OH), and acetic acid (CH3COOH) are known to be protic solvents. These molecules have strongly positively charged hydrogen atoms.
When a hydrogen atom binds to an atom with a high degree of electronegativity, such as an oxygen atom, the hydrogen atom becomes positively charged. As a result, they can form hydrogen bonds. One of the strongest forms of polarization is hydrogen bonding.
Solvents that allow hydrogen bonding are polar protic solvents. In protic solvents, any ion with a positive or negative charge will be stable in the solution.
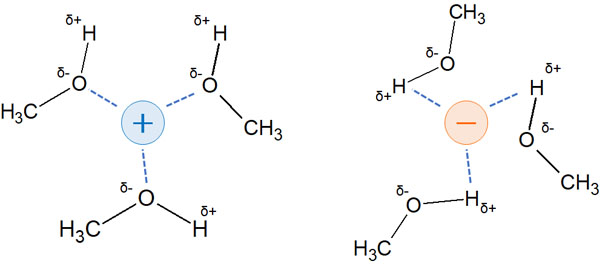
A positively charged molecule will interact with a negatively charged solvent atom. A negatively charged molecule will interact with a positively charged hydrogen atom. As a result, the compound in the solution will be stabilized.
For nucleophiles and leaving groups, acidity and basicity are important for reactivity. Understand that polar protic solvents strongly stabilize ions.
-Polar Aprotic Solvents Weakly Stabilizes Ions
In contrast, there are polar aprotic solvents. Because of the presence of oxygen and nitrogen atoms in the molecule, polar aprotic solvents are polarized. However, hydrogen atoms are not directly bonded to oxygen or nitrogen atoms, and therefore cannot make hydrogen bonds.
These compounds are polar aprotic solvents. The following are known aprotic solvents.
- Acetone
- Acetonitrile
- DMF (N,M-dimethylformamide)
- DMSO (dimethyl sulfoxide)
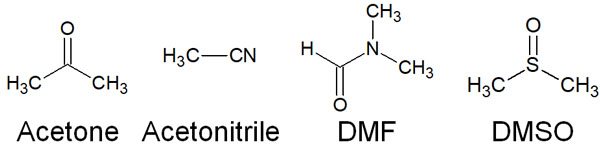
Although there are no hydrogen bonds, these molecules are polarized by oxygen and nitrogen atoms. As a result, they weakly stabilize ions.
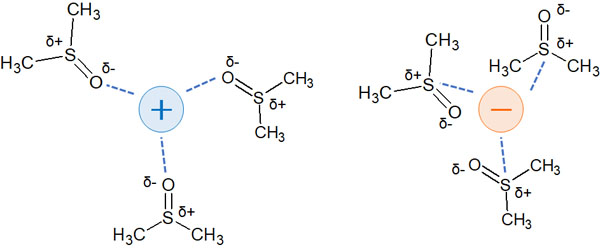
Therefore, consider that the use of aprotic solvents will stabilize the ions of the nucleophiles and leaving groups a bit.
-Non-polar Solvents Do Not Contribute to Stabilization
Non-polar solvents are molecules with only alkyl chains and have no polarity. Examples of non-polar solvents are as follows.
- Hexane
- Benzene
- Toluene
These non-polar solvents are not polarized, so they cannot stabilize either cations (positive charge) or anions (negative charge).
The Ease of SN1 and SN2 Reactions Occurs Vary with Solvents
Since the properties of solvents differ, the reactivity will change depending on the solvent used. For nucleophilic substitution reactions, let’s consider the following.
- SN1 reaction: protic solvents are superior.
- SN2 reaction: aprotic or non-polar solvents are superior.
Why is there such a difference? Let’s start with the SN1 reaction.
The reactivity of the SN1 reaction depends only on the reaction compound. Also, the better the stability of the leaving group, the easier it is to produce carbocation and the higher the reaction rate. After the leaving group is released, a negative ion is produced. In order for this ion to be stabilized, the presence of a polarized solvent makes it more stable.
Also, the carbocation is positively charged, and the use of a polarized solvent stabilizes the cation. For this reason, the SN1 reaction can easily proceed in the following order.
- Protic solvents > aprotic solvents > Non-polar solvents
In an energy diagram, it looks like the following.
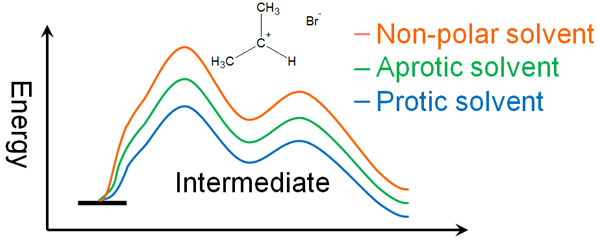
The SN1 reaction is more likely to occur when polar protic solvents are used because the initial activation energy required to produce the intermediate is reduced.
-Solvent Effect in the SN2 Reaction
On the other hand, what about the solvent effect in the SN2 reaction? In the SN2 reaction, the ion of the leaving group is stabilized, so protic solvents seem to be more reactive.
However, it is important to note that in the SN2 reaction, not only the compound undergoing nucleophilic attack but also the nucleophile plays a major role in the reaction rate. The use of protic or aprotic solvents stabilizes the nucleophile. In other words, their ability to attack the compound is weakened.
In the energy diagram, it looks like the following.
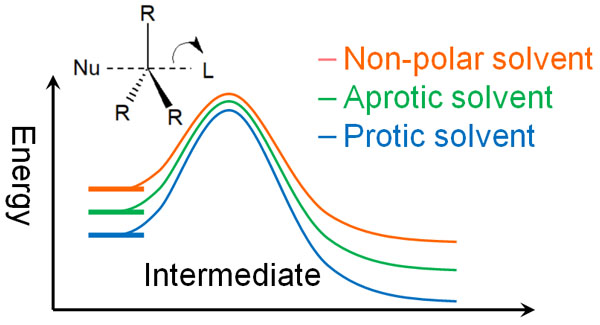
There is no significant difference in peak activation energy. On the other hand, the use of a polarized solvent stabilizes the nucleophile (ion). As a result, the more polarized the solvent, the more activation energy is required for the reaction.
Because more energy is required to enter the transition state, the SN2 reaction does not utilize protic solvents. Aprotic polar solvents or non-polar solvents are commonly used. Polar protic solvents make the SN2 reaction less likely to occur.
Distinguishing the Ease of Nucleophilic Substitution Reactions Occur
One of the most important chemical reactions in organic synthesis is the nucleophilic substitution reaction. It is a convenient synthetic reaction that it can be said that all organic chemists use the SN1 and SN2 reactions. By mixing a compound with a leaving group and a nucleophile, they react chemically.
There are two types of nucleophilic substitution reactions: SN1 and SN2 reactions. Although they are the same nucleophilic substitution reaction, the reactions may be more or less likely to occur under certain conditions. The following factors play a role in these reactions.
- The state of the reacting compound (tertiary, primary, etc.).
- Nucleophile to be used.
- Bonded leaving groups.
- Solvents used in synthetic reactions.
Let’s distinguish between these and consider whether the SN1 or SN2 reaction will occur.
In addition, nucleophilic substitution reactions require you to predict the reactivity and the compounds to be formed, taking into account the stereochemistry. Be sure to understand these reaction mechanisms to synthesize organic compounds.