Compounds with a C=O structure are carbonyl compounds, and there are many types of carbonyl compounds. Among these carbonyl compounds, carbonyl compounds that can be synthesized from carboxylic acids are called carboxylic acid derivatives.
There are different types of carboxylic acid derivatives, such as acyl chloride, acid anhydride, esters, and amides. Each has different reactivity and stability, so the synthetic reactions are different.
However, by understanding the reactivity of each carboxylic acid derivative, you will be able to know what kind of compounds can be synthesized. For carboxylic acid derivatives, nucleophilic acyl substitution reactions are the main reaction, and we can predict what kind of synthetic reaction will occur.
We will explain how to proceed with synthetic reactions using carboxylic acid derivatives, including the reaction mechanisms.
Table of Contents
Nucleophilic Acyl Substitution to Carbonyl Compounds
In organic chemistry, each functional group has its own characteristic synthetic reaction. Nucleophilic acyl substitution reactions occur, especially in the case of carbonyl compounds with leaving groups.
When C=O is attached to an alkyl chain, it is called an acyl group. Acyl groups include many functional groups, such as carbonyl and formyl groups. In short, you can understand that if C=O is present in the molecular structure of the alkyl chain, it is an acyl group.
Among carbonyl compounds, there are several molecules that have leaving groups. These are called carboxylic acid derivatives, and the specific carboxylic acid derivatives are as follows.
- Carboxylic acid
- Ester
- Amide
- Acyl halide
- Acid anhydride
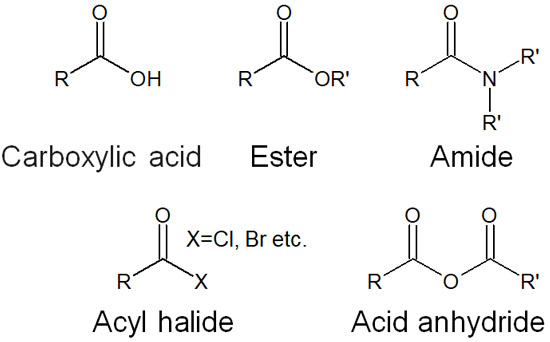
All of these compounds can be synthesized from carboxylic acids.
All carboxylic acid derivatives also have leaving groups. Therefore, nucleophilic agents attack the carbonyl carbon, resulting in substitution reactions. Since the nucleophilic substitution reaction occurs on the acyl group, it is called nucleophilic acyl substitution. The reaction mechanism is as follows.
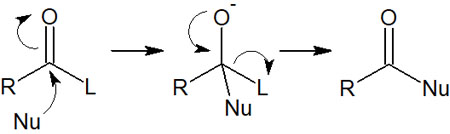
As the nucleophile (Nu) attacks, the leaving group (L) moves away. As a result, a new compound is obtained.
Nucleophilic Addition Reaction in the Absence of Leaving Groups
It is important to note that there are often no leaving groups in carbonyl compounds. Carbonyl groups (ketones) and formyl groups (aldehydes) are examples of this.
In this case, no nucleophilic acyl substitution occurs because there are no leaving groups. Instead, nucleophilic addition reactions take place. Nucleophilic addition reactions have the following reaction mechanism.
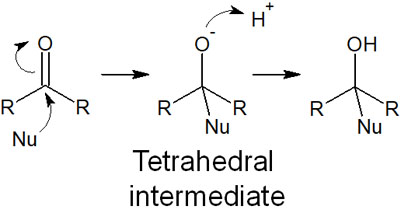
After the reaction, the acyl groups disappear. Understand that when a nucleophilic attack occurs on the acyl group, a different synthetic reaction will proceed in the absence of the leaving group.
The Reaction Mechanism Is Different from the Nucleophilic Substitution (SN1 and SN2)
Nucleophilic substitution reactions, such as SN1 and SN2 reactions, are known to use nucleophilic agents in their synthesis. However, the reaction mechanisms of nucleophilic substitution (SN1 and SN2 reactions) and nucleophilic acyl substitution are different.
In acyl groups, an oxygen atom is attached to a carbon atom. Moreover, since C=O has a double bond, the electrons move, as shown below.
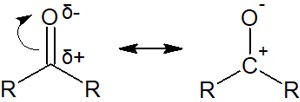
This means that when a nucleophilic reagent attacks the carbonyl carbon, electrons are always transferred to the oxygen atom. Let’s understand that when a nucleophilic attack occurs on an acyl group, the carbonyl carbon is attacked in all synthetic reactions, and then electrons are transferred to the oxygen atom.
Therefore, the carbocation is not produced by the release of the leaving group as in the SN1 reaction. Also, the leaving group does not leave simultaneously as the nucleophile attacks, as is the case in the SN2 reaction. In other words, the following reactions do not occur.
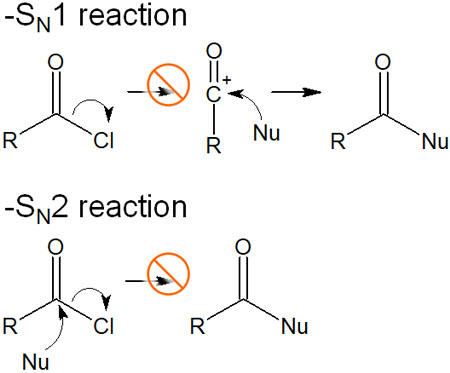
Even though the same nucleophilic reagent is used in the synthesis, the reaction mechanism is different. The movement of electrons changes depending on whether an acyl group is present or not.
Stability (Leaving Capacity) Varies with the Shape of the Carbonyl Compound
What is important when learning about carboxylic acid derivatives? There is an order of reactivity in carboxylic acid derivatives. Learning this order is the most important thing for carboxylic acid derivatives.
The reactivity of carboxylic acid derivatives is in the following order.
-The order of reactivity
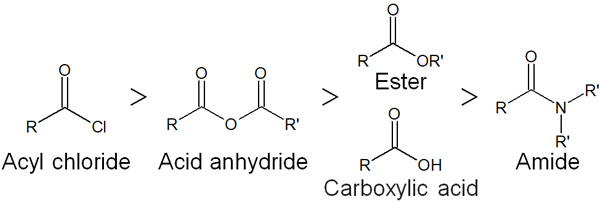
In short, acyl chloride has the lowest stability and is the most reactive. Amides, on the other hand, have low reactivity. For reference, the reactivity of esters is equal to that of carboxylic acids.
Why is the order of reactivity (or stability) different? One reason for this is the resonance structure. For example, in the case of acyl chloride, the chlorine atom has a weak ability to form a double bond. Therefore, the contribution of the resonance structure is weak.
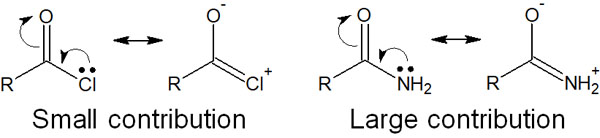
On the other hand, in an amide, the nitrogen atom can make a double bond by pushing out electrons. The contribution of the resonance structure is significant, and the delocalization of electrons makes the molecule stable.
Due to these differences, the reactivity and stability of the carboxylic acid derivative differ.
Consider Reactivity in the Order of Stability
Why is it important to understand the stability of these compounds? It is because we can understand what products will be produced when the compounds are reacted.
For example, when salicylic acid reacts with an acid anhydride, which of the following compounds will you get?
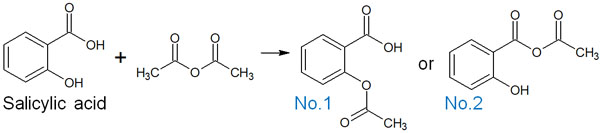
In answer, the compound of No. 2 is not obtained; the compound of No. 1 is synthesized. Compared to acid anhydrides, esters are more stable. Therefore, only the No.1 compound with an ester group can be obtained; the synthesis of the No.2 compound does not proceed because of the presence of acid anhydride in the structural formula.
In the synthesis of compounds, the reaction usually proceeds from an unstable state to a stable state. Therefore, in this reaction, only the compound with the ester group will be obtained.
Of course, it is possible to synthesize highly reactive compounds such as acyl chloride and acid anhydride from carboxylic acids. However, acyl chloride and acid anhydride can only be synthesized under specific reaction conditions, such as the use of special reagents.
Reactivity of Each Carboxylic Acid Derivative
When reacting carboxylic acid derivatives, what is the reaction mechanism? As mentioned above, there are different types of carboxylic acid derivatives. To review, the order of reactivity is as follows.
- Acyl chloride
- Acid anhydride
- Ester and carboxylic acid
- Amide
We will explain how these carbonyl compounds are reacted and synthesized.
Acyl chlorides React with Most Compounds
One of the most beneficial compounds in carboxylic acid derivatives is acyl chloride. The reason why acyl chloride is so important is that it reacts with most compounds.
Acyl chloride can react with carboxylic acids to synthesize acid anhydrides. And if it reacts with alcohols, it can produce esters. And if it reacts with water, it becomes a carboxylic acid. The reaction speed is very fast, and the product can be obtained the moment the reagent is added.

Since it reacts with reagents with very weak nucleophilicity, including water, the synthetic reaction can proceed with any compound after making the acyl chloride.
-Synthesis of Acyl Chloride and Activation of Carboxylic Acid
How do we synthesize a highly reactive acyl chloride? In the synthesis of acyl chloride, the carboxylic acid is usually used. Thionyl chloride (SOCl2), for example, is known as a reagent for reacting with carboxylic acids.
The OH of a carboxylic acid has a low leaving capacity. Therefore, by adding thionyl chloride, the carboxylic acid is activated. The reaction mechanism is as follows.
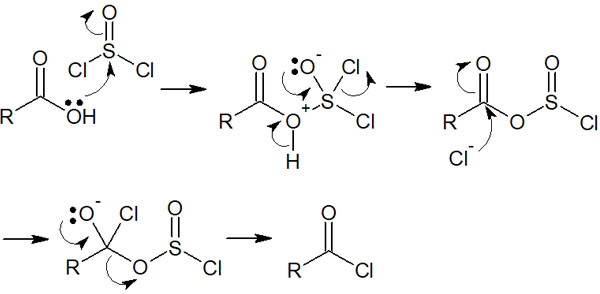
After synthesizing acyl chloride, the next synthetic reaction must proceed immediately by adding a reagent (nucleophile). As mentioned earlier, acyl chloride reacts with water to form a carboxylic acid. Since acyl chloride compounds are difficult to isolate, it is common to proceed with the next synthetic reaction immediately after obtaining acyl chloride.
Acid Anhydrides Are Less Stable and React with Many Compounds
Although they are not unstable as acyl chloride, acid anhydrides have high reactivity and react with a large number of compounds.
Even compounds with low nucleophilicity, such as water and alcohols, which do not have an electrical charge, can be reacted with acid anhydrides. The reason for this is that, as mentioned above, more stable esters and carboxylic acids are formed.
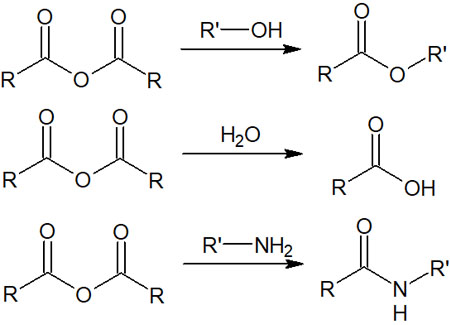
Since amines are highly nucleophilic, they naturally react with acid anhydrides to give amides. However, it is important to understand that they also react with water, which is not highly nucleophilic.
Once you understand the reaction of acyl chloride, it is easy to understand the reaction mechanism of acid anhydrides. In nucleophilic acyl substitutions of acid anhydrides, the leaving group is not Cl–, but COOR.
Reaction Mechanism and Protective Group of Ester
Esters have the structure of COO. The key reaction in the synthesis of esters is hydrolysis. Carboxylic acids are synthesized through hydrolysis by reacting under basic or acidic conditions. In particular, when hydrolysis occurs under basic conditions, it is called saponification.
In the case of hydrolysis under basic conditions, after the carboxylic acid is formed, the proton is withdrawn by the base, and the carboxylic acid takes on a negative charge. Therefore, saponification is an irreversible reaction.

On the other hand, when hydrolysis occurs under acidic conditions, the reaction is reversible. When a carboxylic acid is formed under acidic conditions, the carboxylic acid is not negatively charged.
By reacting with water, the ester is hydrolyzed under acidic conditions as follows.
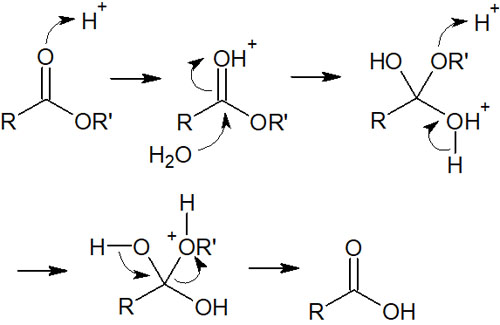
In any case, understand that the esters become carboxylic acids by hydrolysis.
-Esters Are Important as Protecting Groups
Among carboxylic acid derivatives, esters play an important role in protecting groups. Esters can be easily synthesized from carboxylic acids. Not only that, but the hydrolysis of esters to carboxylic acids is also easy.
Esters can be synthesized by combining carboxylic acids with alcohols (hydroxy groups: -OH). Therefore, if there is a carboxylic acid or hydroxy group in the molecule, it is a good idea to use the ester to protect compounds beforehand.
In particular, the carboxylic acid is an acidic compound, which easily produces by-products during synthesis and is difficult to purify after synthesis. For this reason, carboxylic acids are often esterified in advance and hydrolyzed (deprotected) as necessary to obtain the target compound.
-Ester Exchange Yields Other Esters
We explained that hydrolysis and deprotection could be achieved by reacting the reagent with water. If the reagent to be reacted is not water but another alcohol, an ester exchange will occur. In other words, the ester is replaced by another alcohol.
Under acidic conditions, as mentioned above, the ester reaction is reversible. If water is added, the product becomes a carboxylic acid, and if it is reacted with another alcohol, the product becomes another ester.
For example, the methyl ester compound is mixed with an alcohol and reacted at high temperatures. The boiling point of the methanol produced after the ester exchange is low, and the methanol evaporates quickly under high-temperature conditions.
On the other hand, if an alcohol with a high boiling point is present, it will not evaporate as quickly as methanol. For example, methanol has a boiling point of 65°C, while 1-butanol has a boiling point of 118°C. Therefore, if you mix a methyl ester compound with 1-butanol and heat it at around 65°C, it will replace the butanol-derived ester.
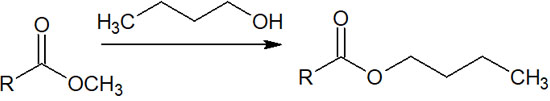
In this way, a synthetic reaction by ester exchange can be carried out under acidic conditions. While removing the generated alcohol, the target compound can be obtained by reacting with other alcohols.
Synthesis of Esters from Carboxylic Acid by Acidic Conditions
With carboxylic acid derivatives, you also have to understand the reaction of the carboxylic acid. In the case of carboxylic acid derivatives, they react with various nucleophiles. It is important to note that carboxylic acids, on the other hand, do not react with nucleophiles.
Normally, the more basic the compound is, the more nucleophilic it becomes. In other words, most nucleophiles are bases. These highly nucleophilic compounds, such as Grignard reagents and amines, are basic.
When a basic substance is mixed with a carboxylic acid, an acid-base reaction takes preference over a synthetic reaction by a nucleophilic attack. The hydrogen atom of the carboxylic acid is pulled out by the base, as shown below.
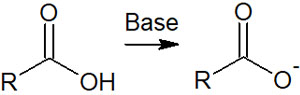
Therefore, it is not possible to perform the reaction using a nucleophile. So, the synthesis is carried out under acidic conditions instead of basic conditions.
Under acidic conditions, carboxylic acids can be esterified. The carboxylic acid is mixed with an alcohol, and the ester compound is obtained by the reaction. The reaction mechanism is the reverse of the synthesis of carboxylic acids from esters under acidic conditions.
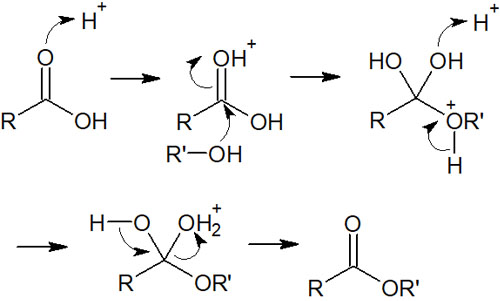
We explained that the hydrolysis of esters is a reversible reaction. Therefore, esters can be synthesized from carboxylic acids in the same way.
Amides Can Be Activated by Acid and Hydrolyzed
Among carboxylic acid derivatives, amides are the stable compounds. In the case of amides, the leaving group will be an amino group. However, the amino group is a highly basic compound and is usually not a leaving group. Although it is possible to synthesize amides by reacting with amines, it is difficult to obtain products from amines.
However, under acidic conditions, the amide can be activated and hydrolyzed.
The amide will not become a leaving group in the -NH2, -NHR, or -NR2 state. On the other hand, if an acid catalyst is added to make it in the -NH3+, -NH2R+, or -NHR2+ state, the amide will be activated. By taking on a positive charge, they become a leaving group.
By adding water or alcohol under acidic conditions, carboxylic acid or ester can be synthesized from the amide.
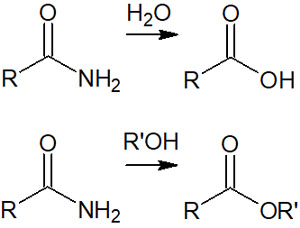
The detailed reaction mechanism is as follows.
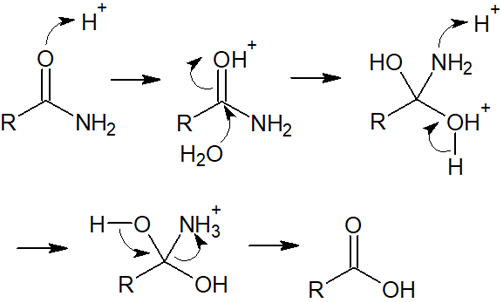
Since the reaction mechanism for the amide hydrolysis is almost the same as that of esters and carboxylic acids, it is simple. The key is that the amine can take on a positive charge when an H+ (proton) is attached to a nitrogen atom.
The presence of an acid catalyst such as hydrochloric acid or sulfuric acid allows the hydrolysis of the amide to proceed. This point is common to the synthetic reaction with carboxylic acids.
Understanding the Characteristics of Each Carboxylic Acid Derivatives
A very important substituent is C=O. However, even if a compound has a C=O structure, the types of synthetic reactions differ greatly depending on the functional group.
We have explained the properties of carboxylic acid derivatives among compounds with a C=O structure. Carboxylic acid derivatives have acyl groups, and the order of reactivity depends on what kind of atoms are attached to them.
Important compounds in carboxylic acid derivatives include acyl chloride, acid anhydrides, esters, and amides. The reaction mechanisms are all similar. However, the reaction conditions and the compounds that can be formed are different. So, let’s understand what kind of reaction conditions are involved in the synthesis of these compounds.
In organic chemistry laboratories, nucleophilic acyl substitution reactions are frequently used synthetic reactions. Therefore, it is necessary to understand the reactivity and reaction mechanism in advance.