The synthetic reaction to create new carbon is one of the most important reactions in organic chemistry. When making new carbon chains, the presence of anions (negatively charged ions) of carbon is very useful. These anion compounds include organometallic compounds.
Because they are anionic carbon atoms, organometallic compounds are strong bases. Anions also act as nucleophiles. Although they are not carbanion, organometallic compounds are highly carbanionic.
So, how do we synthesize organometallic compounds? And how can we use organometallic compounds in synthetic reactions? Why is it necessary to use organometallic compounds?
Learning the properties of organic chemistry will help you to understand these things. We will explain how we can use organometallic compounds to synthesize compounds.
Table of Contents
Organometallic Compounds Allow Carbon to Exist as Anions
There are so many situations where metals are used in organic chemistry. Every researcher in the organic chemistry lab uses metals as catalysts. Among these metals, there are compounds that have metals in their molecules. These are organometallic compounds.
There are compounds in the molecule that can have a negative charge as a carbanion. A typical example is a compound with a triple bond at the end of the molecule (alkyne).
For example, acetylene, an alkyne, is highly acidic. Therefore, when it reacts with a strong base, it produces acetylide.
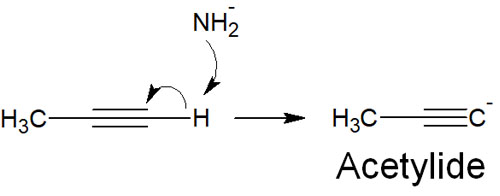
Molecules with sp hybrid orbitals can make carbanion.
On the other hand, what about molecules with sp3 or sp2 hybrid orbitals? In this case, it is difficult to make anions (with a few exceptions).
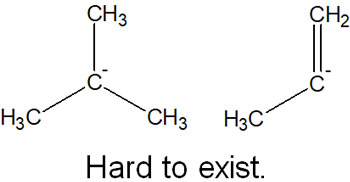
To solve this problem, metals are used. Mg (magnesium) and Li (lithium) are often used as organometallic compounds. In organometallic compounds, a bond between a metal atom and a carbon atom can be created.
When an oxygen or nitrogen atom is bonded to a carbon atom, the carbon atom becomes positively charged. This is because carbon atoms have a lower electronegativity.
On the other hand, Mg (magnesium) and Li (lithium) are on the left side of the periodic table of elements, so carbon atoms have a higher electronegativity. Therefore, contrary to the case when oxygen or nitrogen atoms are bonded, carbon atoms become negatively charged.
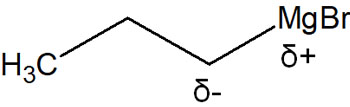
This is why carbon atoms have a negative charge in organometallic compounds. It is not a carbanion because it has a carbon-metal covalent bond. However, the carbon atom is strongly polarized in a negative charge; it can react in the same way as a carbanion.
Grignard Reaction Using Magnesium Is the Most Famous
Grignard reagents are the most famous organometallic compounds that have covalent bonds. Grignard reagents are compounds that have magnesium in their molecules.
Grignard reagents can be made by mixing alkyl halides with magnesium metal in a dry ether solvent. There are various anhydrous ether solvents, such as diethyl ether and THF (tetrahydrofuran).
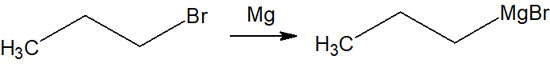
A Grignard reagent can be made if a chlorine, bromine, or iodine atom is bonded to an alkyl chain.
When the molecule reacts on the surface of the magnesium metal, a Grignard reagent is synthesized. However, the surface of magnesium is usually covered with magnesium oxide. Therefore, it is necessary not only to grind the magnesium metal into small pieces but also to remove the magnesium oxide from the surface by using ultrasound.
Since magnesium metal makes covalent bonds, the oxidation state of magnesium is Mg(0) to Mg(II). Therefore, this reaction is called oxidative insertion or oxidative addition. Incidentally, oxidative addition occurs not only with Mg (magnesium) but also with Li (lithium), Cu (copper), and Zu (zinc).
The Grignard reaction was discovered by the French chemist Victor Grignard. For this work, he won the Nobel Prize in Chemistry.
Li (Lithium) to Make Strongly Basic Compounds
While the Grignard reagent is an organic compound using magnesium (Mg), the organometallic compound using lithium (Li) is also well known and widely used in chemical synthesis.
Like the Grignard reaction, lithium metal compounds can be obtained by chemical reaction with alkyl halides. When using metallic lithium, two equivalents of lithium to alkyl halides are required. This is because lithium halide is formed at the same time as the lithium metal compound is formed.
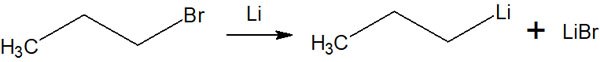
In this case, an anhydrous ether or hydrocarbon solvent is used to react alkyl halides with lithium metal.
As is common to all organometallic compounds, including Grignard reagents, lithium compounds are strong bases. Organolithium compounds, like carbanions, are strong bases.
Cautions in Making Organometallic Compounds: Intramolecular Reactions
So, can we freely make organometallic compounds by reacting alkyl halides with metals? Of course, not. When making organometallic compounds, there are some important points to keep in mind.
What you must consider is the intramolecular reaction. If there is an electrophilic functional group in the molecule, synthetic reactions will proceed within the molecule, at the same time that the organometallic compound is formed. Examples of electrophilic functional groups are as follows.
- Carbonyl group (-C=O)
- Esters (-COO-)
- Cyano group (-C≡N)
In these functional groups, the carbon atom has a positive charge. Therefore, if an electrophilic functional group is present in the molecule, Grignard reagent or organolithium, which are strong bases, nucleophilic attack the carbon atom of the functional group and cause an intramolecular reaction.
The presence of a functional group with a positively charged hydrogen atom is also not allowed. The following functional groups fall into this category.
- Hydroxy group (-OH)
- Amino group (-NH2)
- Carboxylic acid (-COOH)
Due to the strong electronegativity of oxygen and nitrogen atoms, the hydrogen bonded to these atoms is positively charged. Therefore, the hydrogen (proton) is pulled out by reacting with the anionic carbon.
Grignard reagents and organolithium compounds are excellent, but their use is limited. This is because they cannot be used if these functional groups are present in the molecule.
We mentioned that dried ether is used as a solvent for the Grignard reaction because water reacts with organometallic compounds. When using organometallic compounds, the reaction should be carried out in a water-free environment (anhydrous conditions).
Alkylation and Reaction Mechanism in Organometallic Compounds
Why is it important to learn about organometallic compounds? It’s because we can use organometallic compounds to make new carbon chains.
If there is a negatively charged carbon, the nucleophilic attack by that carbon will alkylate the compound and create a new carbon chain. For example, the following synthetic reaction is possible.
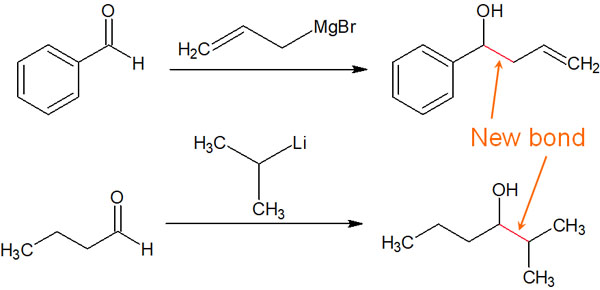
By reacting with a compound containing a carbonyl group (C=O), a new alkyl chain is formed by alkylation.
In many cases, Grignard reagents and organolithium compounds react with molecules containing carbonyl groups. Cationic carbon atoms, which have almost the same properties as the carbanion, attack the carbonyl group. As a result, the molecule can be alkylated.
-Reaction Mechanism of Grignard Reagent
What is the reaction mechanism of organometallic compounds? We will use the Grignard reaction as an example.
As mentioned above, metal atoms have weak electronegativity, so when they bond with carbon atoms through covalent bonds, the carbon atoms become negatively charged. As a result, the carbon atom will nucleophilic attack the carbonyl group. At the same time, the oxygen atom of the carbonyl group coordinates with a metal (magnesium).
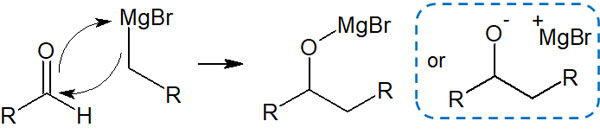
Subsequently, the addition of water eliminates the metal atom attached to the oxygen atom, forming a hydroxy group (-OH).
The detailed reaction mechanism of organometallic compounds, such as the Grignard reaction, has not been understood. In general, however, the reaction mechanism is explained in this way.
-React with Epoxides to Make Alcohols
Since carbon atoms have nucleophilic properties, they can react not only with carbonyl and aldehyde groups but also with epoxides. The most effective reactions with metal compounds are carbonyl groups and aldehyde groups, but epoxides can also be used.
Since organometallic compounds are strong bases, the synthetic reaction with epoxides proceeds under basic conditions. When epoxides are reacted under basic conditions, they nucleophilic attack the carbon atoms of epoxides with fewer substituents.
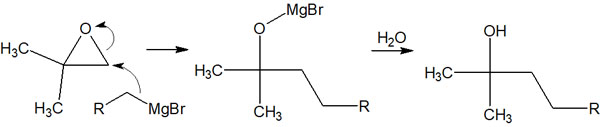
Why are carbon atoms with fewer substitution groups attacked? It is because they have fewer steric hindrances. This reaction mechanism allows epoxides to react with organometallic compounds.
Alcohol Synthesis by Reacting with Ketone or Aldehyde
Chemical reactions with these organometallic compounds create alkyl chains, and at the same time, alcohols are synthesized. Whether it is a nucleophilic attack on a carbonyl or aldehyde group, or a reaction with an epoxide, a compound with a hydroxy group (-OH) is produced.
As mentioned above, reactions with compounds having a carbonyl group (C=O) are important for Grignard reagents and organolithium compounds. Note that depending on whether the compound to be reacted is a ketone or an aldehyde, the resulting compound will differ as follows.
- Reacts with formaldehyde: produces primary alcohol.
- Reacts with aldehyde: produce secondary alcohol.
- Reacts with ketone: produce tertiary alcohol.
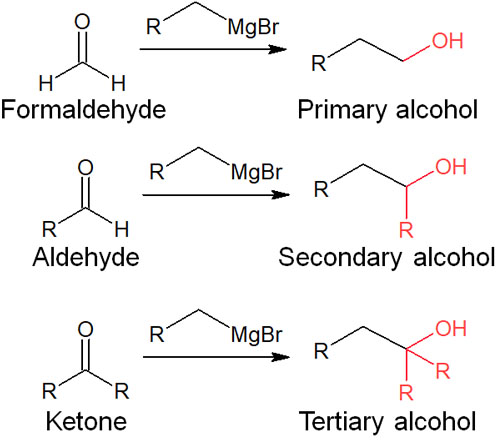
Depending on what kind of reagent is reacted with the organometallic compound, the alcohol synthesized will differ. In any case, the end product is an alcohol. It is important to understand that what kind of an alkyl compound will be synthesized depends on the functional group.
-React with Carbon Dioxide to Obtain Carboxylic Acid
For reference, carbon dioxide (CO2) is known to be a molecule with C=O structures. Since organometallic compounds react with ketones and aldehydes, synthetic reactions also proceed with carbon dioxide, which has C=O structures.
When carbon dioxide reacts with an organometallic compound, a carboxylic acid can be synthesized. The reaction mechanism is as follows.
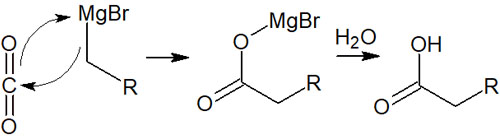
The reaction mechanism for all organometallic compounds is the same. All you have to do is convert the reaction reagent to carbon dioxide, and the end product is a carboxylic acid.
1,2 Addition Proceeding, Michael Addition (1,4 Addition) Does Not Occur
There are some compounds with α,β-unsaturated carbonyls. α,β-Unsaturated carbonyl compounds have the following structure.
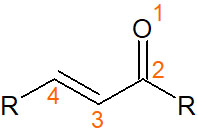
In the case of α,β-unsaturated carbonyls, it is widely known that Michael addition occurs when a nucleophilic attack takes place.
When a nucleophilic reagent attacks the carbons of the carbonyl group, it is called 1,2 addition. On the other hand, when a nucleophilic reagent attacks the carbons of the double bond, it is called Michael addition (1,4 addition).
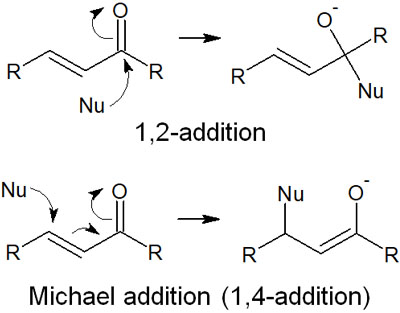
When reacting with carbonyl compounds, there are two places for nucleophiles to attack in the α,β-unsaturated carbonyl. In such cases, Michael addition does not proceed with Grignard reagents and organolithium compounds. In other words, the compound is synthesized by 1,2 addition, not 1,4 addition.
Regioselectivity is important in organic chemistry. Although there are exceptions, such as organometallic compounds using Cu (copper) that proceed by 1,4 addition, in general, organometallic compounds proceed by 1,2 addition.
Organometallic Compounds Are Highly Useful
Any molecule with the structural formula of C=O can be reacted with organometallic compounds to create new carbon bonds. By reacting with aldehydes and ketones, you can get primary, secondary and tertiary alcohols.
Alternatively, it can be reacted with epoxides in a synthetic reaction. You can also react with carbon dioxide to obtain carboxylic acids.
Grignard reagents and organolithium compounds are very famous organometallic compounds. By using organometallic compound reagents, you can make new bonds.
However, not all compounds can be used for organometallic compounds. If there is an electrophilic functional group in the molecule, you cannot make a Grignard reagent because of the intramolecular reaction. In addition to that, you should understand the regioselectivity and try to use organometallic compounds to proceed with the synthetic reaction.