It is important in organic chemistry to change the form of the functional group to get the substituent you want. When you perform a synthetic reaction, you have to synthesize a compound that has the functional group you want.
One of those synthetic reactions is carbonyl reduction. Among the reduction reactions, the reduction of carbonyl compounds is an important synthetic reaction. There are many functional groups with a C=O structure, and we need to learn what kind of functional groups can be reduced.
Regioselectivity is also important. It is common for one compound to have several functional groups, and we need to reduce only certain functional groups.
Depending on the reagents used, the reduction process will be different. So we will explain how to think about carbonyl reduction.
Table of Contents
- 1 Carbonyl Reduction by Hydride Reducing Agents
- 2 Various Reduction Methods for Carbonyl Compounds
- 2.1 Converting Ketones and Aldehydes to Alcohol
- 2.2 Use Lithium Borohydride in the Reduction of Alcohol from Ester
- 2.3 Amide to Amine Synthesis with Lithium Aluminium Hydride
- 2.4 Carboxylic Acid to Alcohol with Borane
- 2.5 Reduction of Ester to Aldehyde Using DIBAL-H
- 2.6 Amides Are Reacted with LiAlH4 Under Low-Temperature Conditions to Obtain Aldehyde
- 3 Differences, Strengths, and Functional Group Selectivity of Hydride Reducing Agents
Carbonyl Reduction by Hydride Reducing Agents
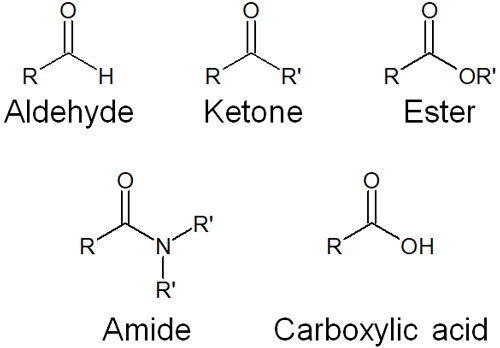
There are many compounds with a C=O structure; molecules with a C=O functional group are called carbonyl compounds. Examples of carbonyl compounds include the following.
- Aldehyde (formyl group)
- Ketone (carbonyl group)
- Ester
- Amide
- Carboxylic acid
Hydride reducing agents are frequently used to reduce these compounds.
Reducing agents that give hydride (H–) are called hydride reducing agents. By mixing the carbonyl compound with a hydride reducing agent, C=O is reduced. As a result, the form of the functional group is changed.
Reduction of Different Carbonyl Compounds in NaBH4 and LiAlH4
There are several types of hydride reducing agents. Specifically, the following types of reducing agents are used in carbonyl reduction.
- Sodium borohydride (NaBH4)
- Lithium borohydride (LiBH4)
- Lithium aluminium hydride (LiAlH4)
- Borane (BH3)
These reducing agents are used. Sodium borohydride (NaBH4) and lithium aluminium hydride (LiAlH4) is particularly important in hydride reduction.
Lithium aluminium hydride (LiAlH4) is highly reactive and reduces most carbonyl compounds. Due to its high reactivity, it ignites when exposed to water. Sodium borohydride (NaBH4), on the other hand, allows the synthesis to proceed under moderate conditions.
The functional groups that can be reduced are different between sodium borohydride (NaBH4) and lithium aluminium hydride (LiAlH4). In the case of sodium borohydride, the reduction of aldehydes (formyl groups) and ketones (carbonyl groups) is possible.
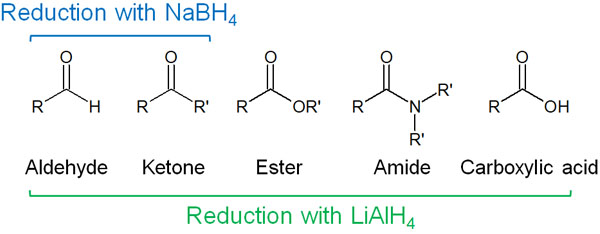
On the other hand, lithium aluminium hydride can reduce most carbonyl compounds. Therefore, it is necessary to use a different hydride reducing agent depending on the compound to be reduced.
-Functional Group Selectivity and Differences Among Hydride Reducing Agents
It is important to note that different hydride reducing agents can reduce different functional groups. By taking advantage of this difference, only specific functional groups can be reduced in a regioselective manner.
For example, suppose we have a compound with ketones and carboxylic acids in the molecule. When sodium borohydride (NaBH4) is added to this compound, only the ketones are reduced. This is because sodium borohydride does not reduce carboxylic acids.
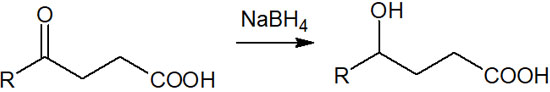
In this way, the molecules can be reduced in a regioselective manner.
Note that we have only discussed the reduction of sodium borohydride and lithium aluminium hydride. However, there are other important hydride reducing agents as well. The functional group selectivity of hydride reducing agents is shown below.
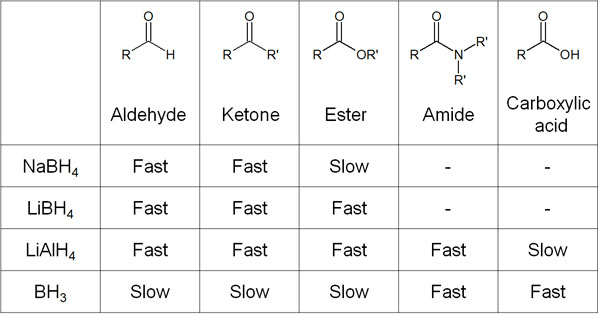
By taking advantage of the differences in the functional groups that can be reduced, it is possible to obtain the desired compound by reducing only the necessary portion.
Hydride Migration Is Important in the Reaction Mechanism
Hydride migration (or hydride transfer) is an important reaction mechanism when using hydride reducing agents. H– is the hydride, and nucleophilic attack by the hydride leads to the reduction of the carbonyl compound. The reagent that causes hydride migration is a hydride reducing agent.
So how does hydride transfer occur? As an example, here is the reaction mechanism when sodium borohydride (NaBH4) reacts with a carbonyl compound in ethanol solvent.
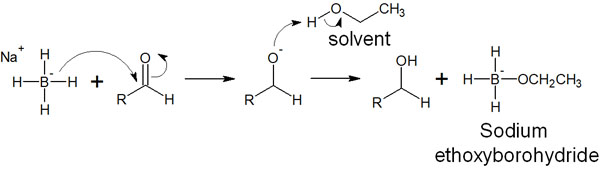
When a hydride reducing agent is added, the H– nucleophilically attacks the carbonyl carbon by hydride migration. At the same time, the electrons forming the C=O double bond are transferred to the oxygen atom. The negatively charged oxygen atom then takes away a hydrogen atom from ethanol. As a result, the compound is reduced to alcohol (hydroxy group: -OH).
Since ethanol loses its hydrogen atoms, ethoxide is formed. The ethoxide then reacts with sodium borohydride, which released the hydride, to form sodium ethoxyborohydride.
Like sodium borohydride (NaBH4), sodium ethoxyborohydride has the ability to reduce carbonyl compounds. Since sodium ethoxyborohydride has three hydrogen atoms bonded to it, it can reduce carbonyl compounds three more times.
Various Reduction Methods for Carbonyl Compounds
How can we reduce carbonyl compounds? As mentioned above, there are many types of carbonyl compounds. For each functional group, you have to choose the best hydride reducing agent.
In the use of hydride reducing agents, it is necessary to use different reagents depending on the functional group to be reacted with. Specifically, consider the following.
- Reduction of ketone or aldehyde to alcohol: use NaBH4
- Reduction of ester to alcohol: use LiBH4
- Reduction of amides to amines: use LiAlH4
- Reduction of carboxylic acid to alcohol: use BH3
- Reduction of esters to aldehyde: use DIBAL-H
- Reduction of amides to aldehydes: use LiAlH4 (low temperature: 0°C)
In the actual organic synthesis, it is necessary to understand the reduction method for each functional group. Therefore, we will explain the reduction method for each functional group.
Converting Ketones and Aldehydes to Alcohol
In the case of compounds with ketones (carbonyl groups) and aldehydes (formyl groups) in the molecule, they can be converted to alcohols using hydride reducing agents.
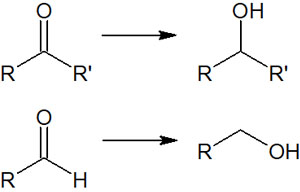
It is known that by oxidizing alcohol, ketones or aldehydes can be synthesized. In other words, by using a reducing agent, the reverse can be done, converting ketones and aldehydes to alcohols.
The most used reagent in the reduction of ketones and aldehydes is sodium borohydride (NaBH4). Lithium aluminium hydride (LiAlH4) can also be used for the reduction, but as mentioned above, LiAH4 ignites when reacting with water or other substances. In addition, LiAH4 produces byproducts when it reacts with other functional groups.
Therefore, NaBH4 is used for selective reduction of ketones and aldehydes. The reaction mechanism is as follows.
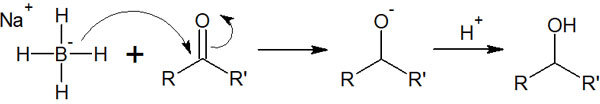
Earlier, we described the reaction mechanism using a hydride reducing agent. This hydride reduction is carried out in exactly the same reaction mechanism.
Use Lithium Borohydride in the Reduction of Alcohol from Ester
On the other hand, how do we reduce an ester to alcohol? For ketones and aldehydes, sodium borohydride (NaBH4) quickly reduces the compound. However, the reaction with esters is extremely slow.
Therefore, lithium borohydride (LiBH4) is used in the reduction reaction of esters. Due to its strong reduction action, even the ester can be easily reduced to an alcohol.
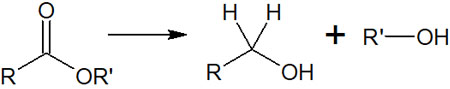
When the ester is reduced by the hydride reducing agent, the reaction mechanism is a little more complicated than the previous one. It is as follows.
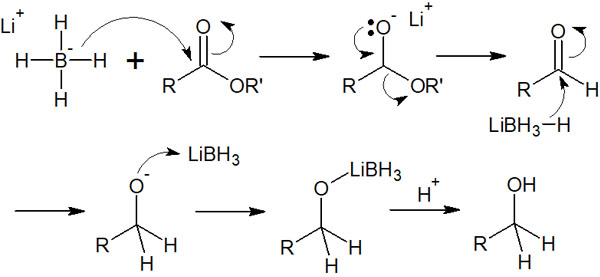
After a nucleophilic attack by hydride transfer, the ester is removed. As a result, the aldehyde is formed.
However, if a hydride reducing agent is present in the solution, the aldehyde will be reduced to an alcohol. Therefore, the ester is eventually converted to alcohol by LiBH4. By reducing the structure of -COOR, it can be converted to -CH2OH.
The ester can also be reduced by lithium aluminium hydride (LiAlH4), but as mentioned above, it reacts with water and explodes, causing a fire hazard. On the other hand, lithium borohydride (LiBH4) is less likely to cause a fire accident than LiAlH4 since the reaction proceeds under moderate conditions.
Amide to Amine Synthesis with Lithium Aluminium Hydride
As a carbonyl compound, a molecule with a stable structure is an amide. A stable structure means that it is difficult to react. Therefore, lithium aluminium hydride (LiAlH4), a powerful hydride reducing agent, is used in the reduction of amides.
By reducing amides, we can synthesize amines. In the reduction of amides, the respective structures can be converted as follows.
- -CO-NH2 → -CH2-NH2
- -CO-NRH → -CH2-NRH
- -CO-NR2 → -CH2-NR2
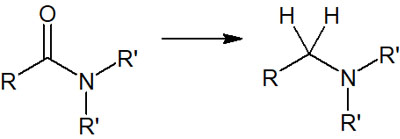
The reaction mechanism is similar to that of an ester. However, unlike the ester, the iminium ion is generated as an intermediate. In the case of esters, we have described that aldehydes are formed in the middle of the reaction by the hydride reducing agent. In the case of amides, on the other hand, the intermediates are iminium ions.
The reaction mechanism of hydride reduction for amides is as follows.
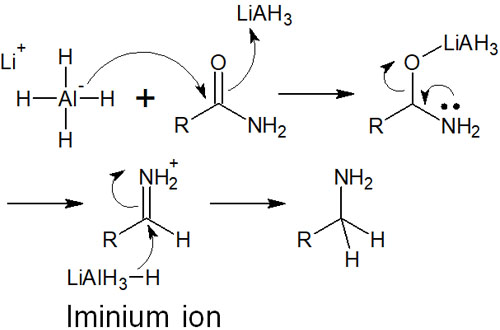
Nucleophilic attack on the iminium ion again results in hydride migration. The result is amine.
Carboxylic Acid to Alcohol with Borane
On the other hand, how are carboxylic acids reduced? Lithium aluminium hydride (LiAlH4) is a highly reactive hydride reducing agent. However, the reaction hardly proceeds when LiAlH4 is added to the carboxylic acid. The reaction of carboxylic acid finally proceeds when the reaction is carried out under high-temperature conditions for a long time.
Why does LiAlH4 react poorly with a carboxylic acid? LiAlH4 is also a base, so the H+ of the carboxylic acid is pulled out by the acid-base reaction. When the reaction by hydride reduction proceeds in this state, dianions are formed as intermediates.
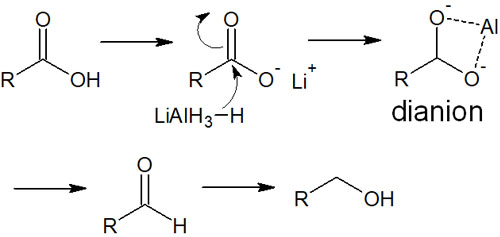
In dianions, two oxygen atoms are coordinated with Al+. This makes the reduction reaction less likely to occur. For these reasons, other reagents are usually used in the reduction of carboxylic acids. Specifically, borane (BH3) is used as a reducing agent.
Borane has an unoccupied orbital and is known to act as a Lewis acid. Therefore, unshared electron pair on the oxygen atom of the carboxylic acid attacks the borane and bonds with the boron atom. Subsequently, the oxygen atom bonded to the boron atom is released.
This is how the reduction reaction proceeds. The reaction mechanism is as follows.
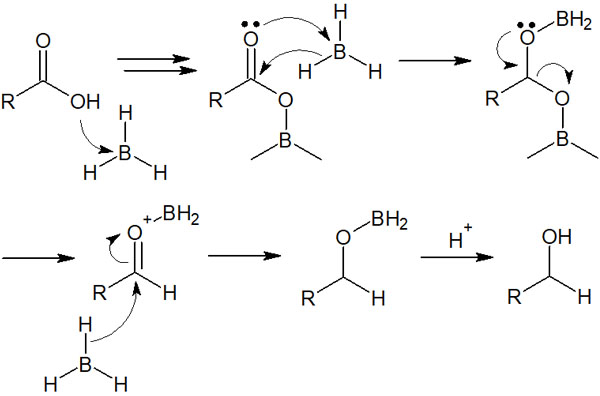
Since borane, which is a Lewis acid, forms a trimer, the reaction mechanism is actually more complicated. However, for the sake of clarity, we have described the reaction mechanism in a simple way, assuming that a single molecule reacts with each other.
Borane can quickly reduce amides and carboxylic acids. On the other hand, it hardly reacts with aldehydes, ketones, and esters. Therefore, it has excellent functional group selectivity.
Reduction of Ester to Aldehyde Using DIBAL-H
So far, we have discussed the reaction of synthesizing alcohols by reduction. To review, the reduction of esters produces aldehydes as intermediates. Is it possible to obtain an aldehyde compound by stopping the reaction at the aldehyde?
With the hydride reducing agents that we have explained, it is impossible to stop the reaction with aldehydes. The general method is to synthesize alcohol and then obtain the aldehyde by an oxidation reaction. However, with some reagents, aldehydes can be synthesized directly from esters.
One such reagent is diisobutylaluminium hydride (DIBAL-H). By reacting with the ester at -70°C, DIBAL-H becomes a stable tetrahedral intermediate.
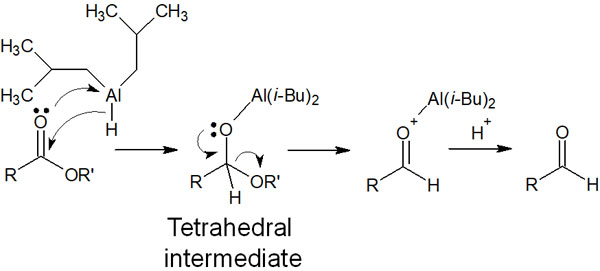
The compound is then decomposed by the addition of water to give the aldehyde. Note that the reaction mechanism of DIBAL-H is similar to that of borane.
Amides Are Reacted with LiAlH4 Under Low-Temperature Conditions to Obtain Aldehyde
There are other methods to synthesize aldehydes by hydride reduction. One of them is the reaction of an amide with lithium aluminium hydride (LiAlH4) at a low temperature (0°C).
As explained in the reduction of amide by LiAlH4, an iminium ion is formed as an intermediate. It is important to note that when an amide reacts with LiAlH4 at a low temperature, the reaction does not proceed to the amine but stops at the formation of the iminium ion.
If water is added to an iminium ion, the compound will be hydrolyzed. The hydrolysis of iminium ion has the following reaction mechanism.
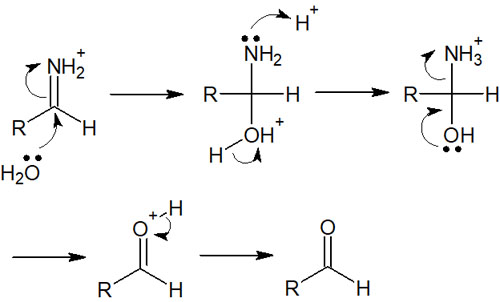
After reacting at 0°C, the iminium ion is converted to an aldehyde by the same reaction mechanism as the hydrolysis of imine.
Differences, Strengths, and Functional Group Selectivity of Hydride Reducing Agents
How to reduce a compound is one of the most important reactions in organic chemistry. When reducing a compound, carbonyl compounds are important. There are many types of carbonyl compounds, such as ketones, aldehydes, and esters, and we must use a hydride reducing agent for each functional group.
Most of the hydride reducing agents contain metals, and their reducing power is different. There are also differences in the types of functional groups that can be reduced and the reduction speed.
By choosing the proper reducing agent, we can selectively reduce the functional group. Typical hydride reducing agents are listed along with their reaction mechanisms, so you should try to understand what kind of reduction agent to use.
In carbonyl reduction, there are many types of hydride reducing agents to choose from. The reaction mechanisms are also different. This makes it more complicated, but it is an important organic reaction and should be learned in advance.