One of the important reaction mechanisms in organic chemistry is the radical reaction. Radicals are highly reactive intermediates, and new bonds can be formed by using radical reactions.
Radical reactions are a different reaction mechanism from the general organic synthetic reactions with acids and bases. It is difficult for many people to understand a radical reaction because we learn something new with different properties.
However, the conditions under which radical reactions occur are fixed. Also, there are certain substances, such as halogens and peracids, that can produce radicals. What’s more, the types of radical reactions are common. Therefore, if you learn when radical reactions occur, you will be able to understand radical reactions.
In this section, we will explain the basics of radical reactions and check how radical reactions proceed to obtain the products.
Table of Contents
The Difference Between Heterolysis (Ion) and Homolysis (Radical)
In general, synthetic reactions in organic chemistry focus on the movement of ions. The relationship between an acid and a base causes the movement of electrons, which causes a synthetic reaction to occur.
When electrons move, two electrons are transferred in most cases. This transfer of electrons is called heterolysis. Heterolysis results in the formation of ions.
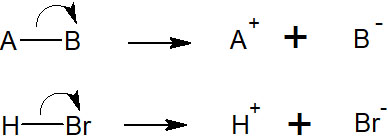
By forming ions, the compound will take on a positive or negative charge. This is the relationship between acids and bases, and heterolysis is involved in most synthetic reactions.
In radical reactions, on the other hand, a phenomenon is called homolysis. When a bond is broken, two electrons do not move to form an ion. Radicals are created when the bond cleaves so that the electrons move one by one to each atom.
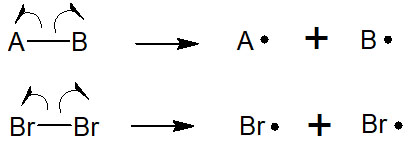
In radical reactions, homolysis is always the first step. Just as acids and bases carry out most synthetic reactions, radical reactions start with radical formation. Therefore, if homolysis does not occur, a radical reaction cannot occur.
Radicals Are Highly Reactive and Give Different Compounds
Among the intermediates of organic compounds, radicals are known to be highly reactive. Because they have one electron instead of two, they can easily react chemically with other compounds.
Also, compared to the ions produced by heterolysis, the radicals produced by homolysis can yield different products. For example, in the addition reaction of hydrogen bromide, the acid/base reaction products and the radical reaction are different as follows.
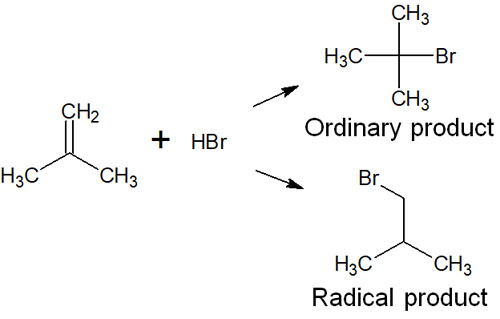
Different reaction conditions will result in different compounds. Radical reactions are important in organic chemistry because they give a different product than reactions with ions.
So why are the products different as described above? In the addition reaction of hydrogen bromide to an alkene, a carbocation is formed, and the synthetic reaction proceeds.
On the other hand, when hydrogen bromide is reacted with the same alkene, radical products can be synthesized in the presence of oxygen or peroxide. Radical reactions mainly use peracids. The peracids create radicals by causing homolysis, which results in radical products.
-Conditions for Obtaining Radicals
When are radicals produced? In most cases, radicals are formed at high temperatures above 200°C by homolysis. Radicals are created when high energy is applied.
However, there are cases where radicals can be produced even if the temperature is a little higher than room temperature. In compounds with weak bonds, radicals are created when the bonds are broken. As mentioned above, we mainly use peracids in radical reactions, and peracids are known to easily produce radicals.
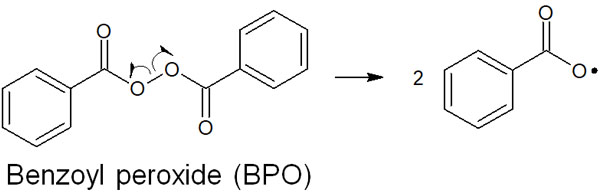
Hydrogen peroxide is one of the most famous peracids. Among these peracids, benzoyl peroxide (BPO) is frequently used as a radical initiator. This is because the bond between oxygen is weak, and radicals are easily generated.
Halogens such as chlorine (Cl2) and bromine (Br2) are also frequently given as examples of radical reactions. When halogens are exposed to heat or light (ultraviolet/UV), the bonds are cleaved, and radicals are formed.

Halogens are also known to bond weakly with each other. Therefore, when halogens such as chlorine and bromine are present, radicals are created.
Understanding the Types of Radical Reactions
What are the different types of radical reactions? We have already discussed homolysis. In all radical reactions, homolysis occurs first. Homolysis is the reason why radical reactions proceed.
There is also the reverse of homolysis. When radicals react with each other and form a bond, it is called radical coupling. The following reaction is radical coupling.
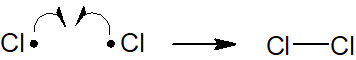
Among the radical reactions, homolysis and radical coupling are easy to understand. However, there are three other types of radical reactions that must be learned. These are the following radical reactions.
- Radical hydrogen abstraction
- Radical addition to a double bond
- Alkene formation in β-cleavage
Learning about these reaction mechanisms will help you to understand how radical reactions occur.
Radical Atom Abstraction Causes Hydrogen Atom Abstraction
One reaction that is characteristic of radicals is the withdrawal of hydrogen atoms. In short, in the presence of radicals, hydrogen atoms are pulled out, and new radicals are formed.
This reaction is known as radical atom abstraction. The most important type of atom abstraction is the hydrogen atom abstraction. For this reason, radical atom abstraction is also called hydrogen atom abstraction.
Why are there two different names for it? Because not only hydrogen but also halogen atoms such as chlorine and bromine can be pulled out. In other words, radical atom abstraction is not limited to hydrogen atom abstraction.
However, if no halogen is present, only hydrogen atom abstraction will occur. Therefore, hydrogen atom abstraction is the most important in radical atom abstraction.
Radical Addition Reaction to Double Bond Is Important
When there is a double bond in a molecule, radicals react with alkenes to cause an addition reaction. The following two points are important in the radical addition reaction to a double bond.
- Reacting with the end of the double bond to create a bond.
- A new radical is created on the opposite side of the added site.
A polysubstituted alkane is synthesized in a normal addition reaction to an alkene, as mentioned in the hydrogen bromide example earlier. On the other hand, in the radical reaction, the radical reacts with the terminal part of the alkene to form a new bond.
After the addition reaction, a new radical is formed on the opposite side. For example, it is as follows.
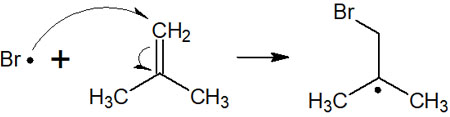
Why do radicals attack the terminal carbon atom? This is because the stability of the radical intermediate is involved.
In general addition reactions, the stability of the carbocation is involved. In the same way, radical intermediates have an order of stability. They are as follows.
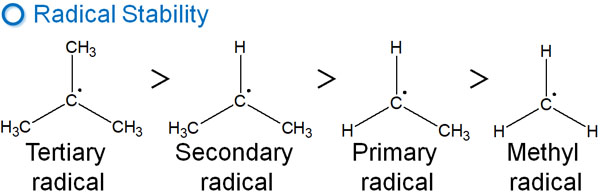
When new radicals are formed on the other side after a radical reaction, they tend to be more stable if they are tertiary or secondary radicals. Although radicals are highly reactive intermediates, they have an order of stability. Therefore, in the radical addition reaction to the double bond, there is regioselectivity.
In the case of the compound mentioned earlier, the intermediate formed after the radical reaction is a tertiary radical. In this way, we can predict the compound to be synthesized.
β-Cleavage Creates a New Double Bond
In radical reactions, a new double bond may be created. This is called beta cleavage (β-cleavage). We have just described the radical addition reaction to a double bond. The opposite reaction is the formation of an alkene by β-cleavage.
When an atom, such as a halogen that causes a radical reaction is bonded to the beta position of a carbon atom, radical cleavage can occur. In this case, a radical is transferred to the halogen while creating a double bond. The reaction mechanism is as follows.
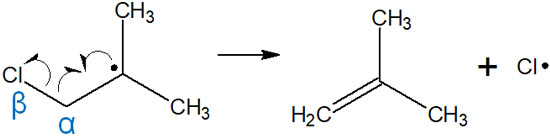
In radical reactions, double bonds can be created by β-cleavage. In this reaction, the radical is transferred to a chlorine atom.
Radical Chain Reaction: Initiation and Propagation Step
There are several types of radical reactions. Importantly, these radical reactions can occur in a chain. In other words, the production of radicals through homolysis leads to a number of radical reactions.
There are always the following stages in a radical reaction.
- Initiation step
- Propagation step
- Termination step
We will explain how the chain reaction works in the following simple radical reaction.
![]()
-A Radical Reaction Begins in the Initiation Step
When chlorine molecules are used in a radical reaction, they are exposed to heat or light (ultraviolet: UV). Then, radicals are generated. All radical reactions have an initiation step due to homolysis.
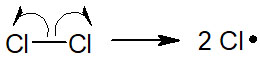
-Obtain Products in the Propagation Step
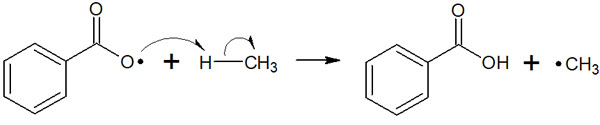
After the radicals are formed, the radicals attack other molecules, which leads to further reactions. In this reaction, the chlorine radical produces a radical in methane by hydrogen atom abstraction. It is as follows.
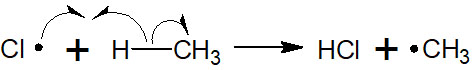
After producing a methyl radical, the radical next attacks the chlorine. This attack yields the desired compound, chloromethane.
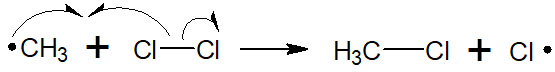
It is important to note that radicals are still being generated even after the target compound is obtained. The radicals generated in this reaction cause radical atom abstraction again, producing methyl radicals. This is called a chain reaction because the reaction occurs one after another.
In this reaction, hydrogen is pulled out by radicals. On the other hand, when an alkene is reacted, for example, an addition reaction to a double bond occurs. The product will differ depending on the type of reagent used in the reaction.
Termination Step Creates Bonds and Stops the Reaction
So, does the chain reaction continue forever? Of course not. Although the chain reaction occurs due to homolysis, the reaction stops at some stage. This is called the termination step.
Radicals cause hydrogen abstraction, addition to double bonds, and beta cleavage. However, radicals often react with each other to form new bonds. Specifically, the reverse reaction of homolysis occurs. The following reactions are examples of this.
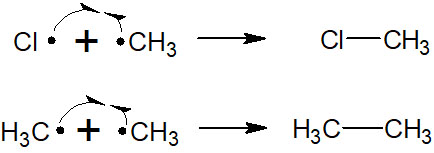
When these reactions occur, the radicals disappear. There is no new homolysis, and the reaction is completed with the creation of new bonds. Radicals make bonds with each other, and the chain reaction is stopped by the termination step.
Strictly speaking, the reaction with oxygen in the air and water is also a radical termination reaction. However, let’s not consider the reaction with air or water and think of the termination step as a radical reaction between compounds.
-A Certain Amount of Radical Reaction Initiator Is Needed
Since the reaction proceeds by a chain reaction, the desired product can be obtained even with a small amount of an initiator such as benzoyl peroxide (BPO).
However, if the amount of initiator is too small, the reaction will not proceed. This is because some of the generated radicals can form new bonds with each other and stop the reaction, as described above. Therefore, the best amount of initiators is not too much or too little.
Reacts with Alkyl Chains to Create New Bonds
How can we use these radical reactions in organic chemistry? Synthetic reactions using radical reactions are frequently used to create new bonds.
For example, by reacting an alkane with a halogen (Cl2 or Br2), we can synthesize a compound with an added halogen.
New carbon bonds can also be created by reacting a compound with a double bond. There are several ways to make carbon chains in organic chemistry. One of them is the radical reaction. For example, new carbon chains can be made by the following synthetic reactions.
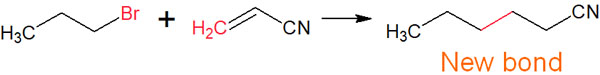
Radical reactions are useful when you want to make new bonds. Radical reactions can be used to add halogens to carbon chains or to create carbon chains.
-Polymers Are Obtained Through Polymerization Reactions
In addition, because the reaction proceeds through a chain reaction, some compounds often undergo a polymerization reaction to yield polymers. Polymers are produced by the formation of carbon chains one after another.
For reference, in compounds that generate radicals by heat or light (UV), radical terminators such as antioxidants are often added. This is because, without the terminator, molecules would cause radical reactions to occur and polymerize to form polymers.
Regioselectivity and Radical Stability
In addition, when using radical reactions, we must consider regioselectivity. It is important to understand which part of the alkyl chain reacts with the radical and what kind of product is obtained.
We have already discussed regioselectivity. The stability of the radical intermediate greatly influences regioselectivity. Again, the order of radical stability is as follows.
- Tertiary radicals > Secondary radicals > Primary radicals
Therefore, when radicals are formed, the formation of radicals with more substituents is given priority. For example, the following is an example.
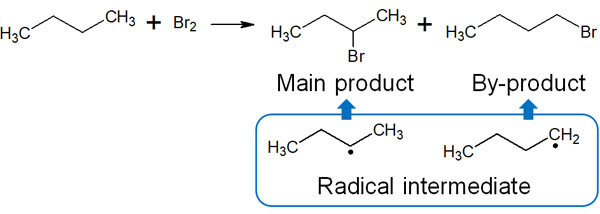
Checking the radical intermediates, the more stable secondary radicals are preferentially produced in this case. Therefore, there is one main compound.
Stability of Allyl and Benzyl Compounds
Allyl and benzyl compounds also exist, depending on the compound. If these compounds have radicals, the structural formula is as follows.
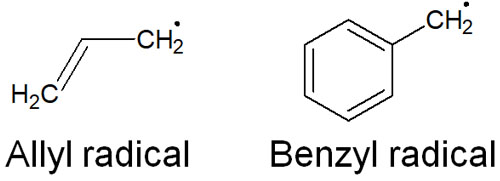
The intermediates of allyl and benzyl radicals are highly stable. Of course, because they are radicals, they are highly reactive. However, allyl and benzyl radicals are known to be more stable than tertiary radicals. The stability of radicals is as follows.
- Benzyl radicals > Allyl radicals > Tertiary radicals > Tertiary radicals > Secondary radicals > Primary radicals
The reason for this order is that we can write the following resonance structures for allyl radicals and benzyl radicals.
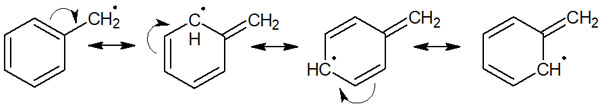
When you understand these, you will be able to understand how the reaction proceeds according to the regioselectivity. For example, in the following reaction, we can consider two types of reactions.
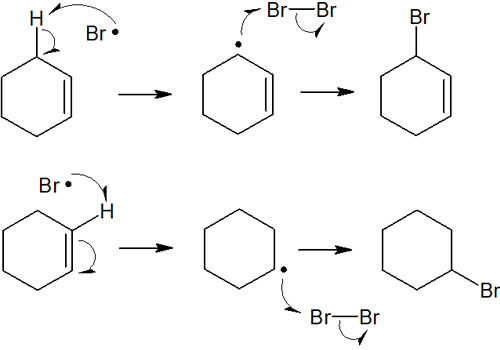
However, there is only one reaction that actually occurs. In the intermediate, you can draw an allyl radical or a secondary radical.
In radicals, the benzyl radicals are more stable. Therefore, the product is 3-bromocyclohexene. Of the compounds shown in the figure above, the upper compound (3-bromocyclohexene) can be obtained selectively. This is the regioselectivity in the radical reaction.
Synthetic Reactions with Highly Reactive Unpaired Electrons
Many people are not familiar with radical reactions because they are not common chemical reactions. Radicals are unstable intermediates with unpaired electrons, and they cause chain reactions.
However, radical reactions are useful for creating new bonds. Nucleophilic substitution, addition, and cleavage reactions are known to be caused by the relationship between acids and bases. Radical reactions are used to obtain compounds that cannot be synthesized by these chemical reactions.
In radicals, the reaction is started by the presence of an initiator that causes homolysis, such as a halogen or peracids. In addition, there are fixed reactions that occur, such as radical atom abstraction and addition to double bonds. Radical intermediates have an order of stability and also regioselectivity.
Once you know these properties, you will be able to understand what kind of compounds can be obtained by radical reactions.