When understanding the synthetic reaction mechanism of organic chemistry, an important factor is the stability of the carbocation. Since carbocation is an unstable substance, it reacts quickly. However, the stability of carbocations varies depending on the molecular structure.
It is very important to understand this difference, because if the stability of the carbocation changes, the reaction that occurs will be different.
This is due to the phenomenon called hyperconjugation. Understanding this phenomenon also allows us to predict the stability of radicals and carbanions.
A carbocation is one of the intermediates in synthetic reactions. Here, we will learn about the stability of carbocation, and then we will explain the stability of other intermediates such as radicals, carbanions and aryl cations.
Table of Contents
Tertiary, Secondary and Primary Carbocation Related to Stability
An important intermediate is a carbocation. A carbocation is often found in organic chemistry and is easy to understand among the intermediates. Therefore, we will first discuss the properties of the carbocation.
The stability of carbocation depends on the substituents in the molecule. It is as follows.
- Tertiary > Secondary > Primary
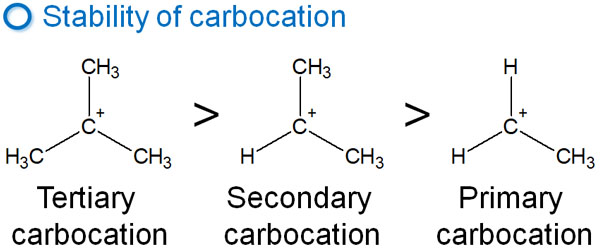
The stability of a carbocation depends on how many alkyl chains (or hydrogen atoms) are attached to a positively charged carbocation.
Many people look at this order and try to remember it without understanding why; they move on to the next step.
But it’s important to understand the nature of the intermediates and the resonance structure of the carbocation; it’s also involved in major organic synthesis reactions, including the SN1 reaction. So make sure you learn why they are in this order.
Carbocations Become Planar in sp2 Hybrid Orbitals
Why does the stability of the carbocation differ depending on the molecular structure? This is related to a phenomenon called hyperconjugation. Hyperconjugation in carbocations can be expressed in simple terms as follows.
- A phenomenon in which the electrons involved in a bond give electrons to a positively charged carbon atom.
What is the molecular shape of a carbocation? Carbocations have sp2 hybrid orbitals. All compounds that form 120° bond angles with each other have sp2 hybrid orbitals. It looks like this.
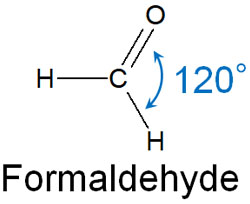
In a carbocation, there are three bonds to a carbon atom. Each of these bonds is located at the most distant point. This results in a bond angle of 120°.
At the same time, in the sp2 hybrid orbital, each atom is located on the same plane. In carbocation, all the atoms bonded to the cation carbon are on the same plane.
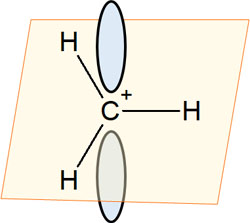
This structure is important for understanding hyperconjugation. The carbocation also has an unoccupied orbital. Since there is an unoccupied orbital (p-orbital) that can hold two electrons, it is common to draw orbital above and below the positively charged carbon atom to explain hyperconjugation.
Hyperconjugation Causes Electrons to Delocalization
What if the bond to the carbon atom is not a hydrogen atom, but a methyl group (CH3)? In this case, the result is as follows.
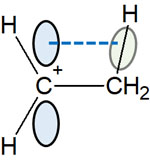
An unoccupied orbital extends vertically from the carbocation, as mentioned above. This p-orbital interacts with the C-H bond of the neighboring methyl group. This is hyperconjugation.
By sharing electrons, carbon and hydrogen are bonded together. This single bond is called the σ bond (sigma bond). However, if there is an atom that lacks electrons, such as a carbocation, the electrons that form the sigma bond will share electrons with the carbocation.
The p-orbital of the carbocation and the C-H bond of the methyl group are parallel. As a result, the electrons forming the σ bond (C-H bond) share electrons with a positively charged carbon atom.
The carbon atom acts to push the electron out. The alkyl chain acts as an electron-donating group. Therefore, although the force is weak, the cation is stabilized by the influence of the carbon atom next to the carbocation.
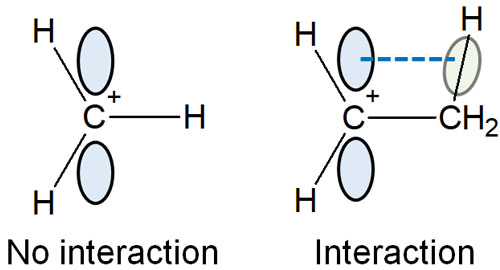
On the other hand, what if there is no alkyl chain (such as a methyl group) next to it? In the case of a carbocation in which hydrogen atoms are directly bonded to a carbon atom, there are no orbitals that overlap parallel to the p-orbital. Therefore, there is no hyperconjugation, and the cation is not stabilized.
Also, the more alkyl chains there are next to each other, the more electrons forming the C-H bond (electrons in the σ-bond) share electrons with the carbocation. As a result, the carbocation becomes more stable. This is why the stability is highest for tertiary carbocations.
The wider the range of electrons, the more stable they are. This is called the delocalization of electrons. The more electrons in the C-H bond interact with the carbocation through hyperconjugation, the more electrons are delocalized, and the more stable the structure becomes.
In fact, there are no methyl cations. Also, primary methyl cations do not form spontaneously. On the other hand, a few secondary carbocations are produced. Tertiary carbocations are more likely to be formed due to their higher stability.
One of the most important synthetic reactions in organic chemistry is the SN1 reaction, in which the carbocation is formed first, and then the chemical reaction takes place; in the SN1 reaction, the formation of the tertiary carbocation (or secondary carbocation) is important. The reason for this is the high stability of the tertiary carbocation.
Interacting with Unoccupied p-orbital in the Plane
Since the carbocation exhibits hyperconjugation, the more alkyl chains are attached to the carbocation, the more stable the cation becomes. However, there is a condition for this: an unoccupied p-orbital must be able to interact with electrons.
In a carbocation, an unoccupied p-orbital is always created. When an alkyl chain is attached, the p-orbital of the carbocation and the C-H bond can overlap in parallel, resulting in hyperconjugation. In contrast, if an unoccupied p-orbital and electrons cannot interact in parallel, even a tertiary carbocation is not stable.
For example, no carbocation is formed in the following compounds.
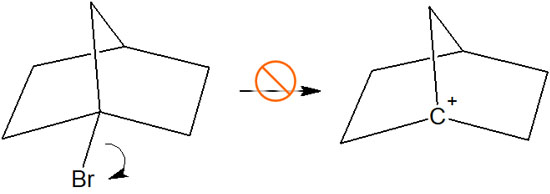
Why doesn’t this compound form in spite of the tertiary carbocation? In the case of this compound, even if it becomes a carbocation, the unoccupied p-orbital and the adjacent C-H bond are not parallel. Therefore, the carbocation is not stable.
In the same way that methyl cations are not formed, if hyperconjugation does not occur even in tertiary carbocations, no cation is produced. The reason why carbocations are stabilized is to understand that the electrons of the single bond (σ bond) and the carbocation weakly share electrons.
Radical Stability Is the Same As Carbocation
In the same way, we can understand the stability of other intermediates as well. In organic chemical reactions, radicals may be produced. What is the three-dimensional structure of radicals? In radicals, there are three bonds, like carbocation, which are sp2 hybrid orbitals.
Unlike carbocation, radicals have one unpaired electron. However, although their properties are different from those of carbocation, the stability of radicals is the same as that of the carbocation. The stability of radicals is as follows.
- Tertiary > Secondary > Primary
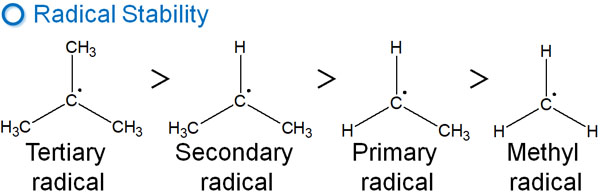
The reason for this order of radical stability is the effect of hyperconjugation. In other words, the stability of radicals can be explained by exactly the same reason as the stability of carbocations.
Radicals, like carbocation, are also lacking in electrons. As a result, their order of stability is the same as that of the carbocation.
The Stability of the Carbanion Is Reversed
If we consider the delocalization of electrons, we can understand the stability of carbocations and radicals. On the other hand, what is the stability of carbanions?
Carbanion has a negative charge. Therefore, their properties are completely different from those of carbocation and radicals, and they do not exhibit hyperconjugation. In addition, compared to carbocation and radicals, the stability of carbanions is reversed as shown below.
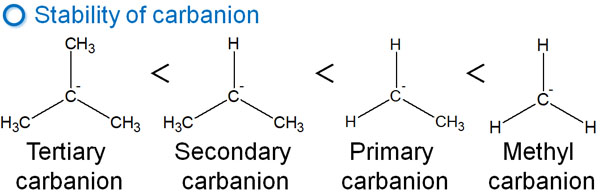
The reason for this can be explained by the delocalization of electrons in the same way.
As mentioned earlier, carbon acts as an electron-donating group. When dispersing the electrons of a carbanion, if there is a carbon atom bonded next to it, the electrons of the anion cannot be dispersed. Rather, the electrons are pushed out by the neighboring carbon atoms, resulting in a higher electron density. As a result, the molecule becomes unstable.
Comparing methyl anion and tertiary carbanion, the electrons in methyl anion are more widely distributed and can be delocalized. On the other hand, tertiary carbanions have a higher electron density.
-Inductive Effect Occurs and Stabilizes Depending on the Degree of Electronegativity
For reference, what if the hydrogen atoms in the methyl anion are halogens? In this case, the electrons of the methyl anion are more widely dispersed by the high electronegativity halogen. This delocalization makes the methyl anion more stable.
The difference in charge in the same molecule caused by electronegativity is called the inductive effect—the inductive effect results in different stability of the anion intermediate.
The principle is different from that of stabilization by hyperconjugation. However, the principle is the same as that of carbocation, in which the stability is changed by the delocalization of electrons.
Stability with Double Bonds (Aryl Cations) and Aromatic Rings (Benzyl Cations)
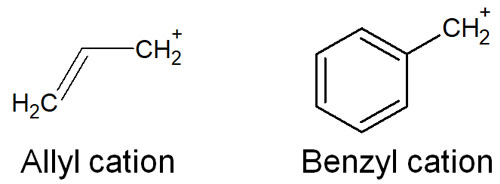
Once you have learned about the stability of these intermediates, you should then try to understand the stability of compounds with a conjugated structure. Once you learn that the delocalization of electrons is important to the stability of the intermediates, it is easier to understand the stability of allyl cations and benzyl cations.
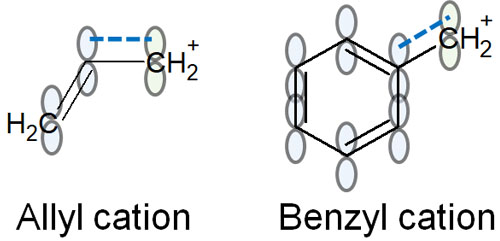
The allyl cation has a double bond. In benzyl cations, there is a benzene ring. Compounds with the following structures are allyl cations and benzyl cations.
Double and triple bonds involve π bonds; in π bonds, the orbits are known to extend up and down. The first single bond is always a σ bond. In addition, when making a double or triple bond, the electron orbitals are stretched up and down, and the bond is formed by the overlap of the electron orbitals.
The bonding strength of the π bond is weak because it is forcing the bond to form.
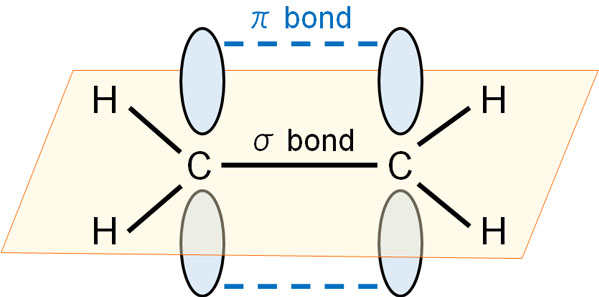
However, due to the presence of π-orbitals above and below, it can share electrons with the unoccupied orbitals of carbocations.
You can also write resonance structures for allyl and benzyl compounds. For example, for a benzyl cation, it looks like the following.
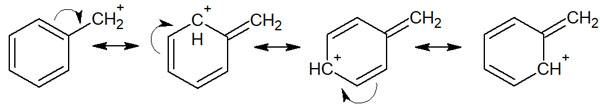
A double bond (alkene) or an aromatic ring (benzene ring) allows us to write resonance structures. As a result, both aryl cations and benzyl cations can be stabilized. The cation is not present in only one place, but the delocalization of electrons carries a positive charge in many places. As a result, the molecule is stabilized by resonances.
Primary carbocation does not form spontaneously. However, even if they are primary carbocation, they are exceptionally stable if you can write resonance structures. Therefore, aryl cations and benzyl cations are formed.
For example, when comparing a secondary carbocation with a primary aryl cation, the primary aryl cation is more stable.
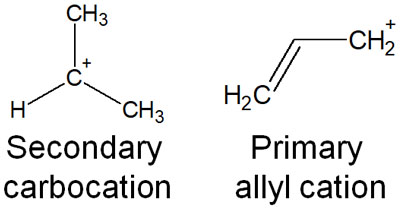
Because there are fewer alkyl chains attached, primary allyl cations are less affected by hyperconjugation. However, because of the resonance effect, the structure is more easily stabilized.
The Allyl and Benzyl Radicals Are Equally Stable
Exactly the same thing can be said for radicals. Allyl radicals and benzyl radicals are more stable. Here are the structural formulas for allyl and benzyl radicals.
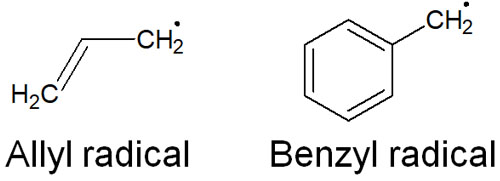
Allyl radicals and benzyl radicals can resonate. For example, the following is the resonance structure of the benzyl radical.
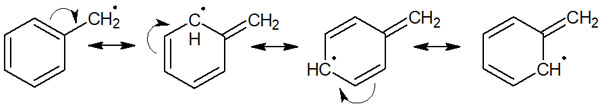
The presence of a conjugated structure due to double bonds allows them to be resonance structures and take form a stable structure. As a result, the delocalization of electrons stabilizes the allyl and benzyl radicals.
Of course, even though they are stable, carbocation and radicals are still highly unstable and are highly reactive intermediates. However, when considering the ease of formation of intermediates, it is important to understand their stability.
In any case, it is important to understand that radicals have a certain order of stability for the same reason as carbocation.
In the case of radicals, the order of stability is as follows.
- Benzyl radicals > Allyl radicals > Tertiary radicals > Secondary radicals > Primary radicals
In the case of radicals, stability is strongly related to the delocalization of electrons by resonance. Also, benzyl radicals with aromatic rings can write more resonance structures than allyl radicals. This delocalization of electrons increases the stability.
As a reminder, this order is not true for carbocation. Just understand that with radicals, the stability will change depending on this order.
-Allyl and Benzyl Anions Are Also Stable
For reference, for allyl and benzyl compounds, the anions are also stable. When a negatively charged carbon is generated, it is more likely to be stable if there is a double bond (alkene) or benzene ring next to it.
The reason for this is the same as for cations and radicals: they can make resonance structures. It is important to understand that the resonance structure allows electrons to exist in many different places, which increases the stability of the molecule.
Stability of Intermediates Is Involved in Synthetic Reactions in Organic Chemistry
When predicting how a compound will react, you need to understand how stable the intermediate products are. The more stable the intermediates are, the easier it is to produce them. This means that the organic synthesis reaction can proceed more easily.
Although there are several intermediates, typical intermediates are carbocations, carbanions and radicals. In particular, carbocations are involved in the SN1 reaction, an important synthetic reaction.
When understanding the stability of carbocations and radicals, it is important to understand the following.
- The tertiary is stable.
- Allyl compounds (with a double bond next to them) are stable.
- Benzyl compounds (with a benzene ring next to them) are stable.
In carbanions, the tertiary is unstable. Therefore, the order of stability must be reversed. However, in carbanions, it is common for allyl compounds, which are alkenes, and benzyl compounds, which have an aromatic ring next to them, to be more stable.
We have explained why these intermediates show stability in a particular order. Let’s learn about these reasons and understand the reaction mechanism of organic synthesis.