The benzene ring is very important in organic chemistry. Compounds with a benzene ring are called aromatic compounds.
The benzene ring contains a lot of electrons. This is the so-called electron-rich state, where the electrons in the benzene ring can react with other molecules to cause an organic chemical reaction. This is called an electrophilic aromatic substitution reactions.
When an electrophilic substitution reaction occurs in the benzene ring, the position where the chemical reaction occurs is fixed. This is called orientation. In more detail, the orientation of an aromatic compound changes depending on the substituent group.
In addition, the reactivity (how efficiently the reaction occurs) also differs depending on the functional group. Here, we will explain how orientation and reactivity in electrophilic aromatic substitution reactions differ depending on the substituents.
Table of Contents
- 1 Aromatic Electrophilic Substitution Reactions Take Place in Different Places
- 2 The Orientation of Benzene Rings Varies with Electron-Donating and Electron-Withdrawing Groups
- 3 Halogens (Chlorine and Bromine) Have ortho- and para-Orientation
- 4 Alkylbenzene (Toluene) Has ortho-para Orientation
- 5 Reactivity Varies Greatly with Electron-Donating and Electron-Withdrawing Groups
- 6 Understanding Electrophilic Aromatic Substitution Reactions
Aromatic Electrophilic Substitution Reactions Take Place in Different Places
When an electrophilic substitution reaction occurs on a benzene ring, the substitution site differs depending on the type of aromatic compound.
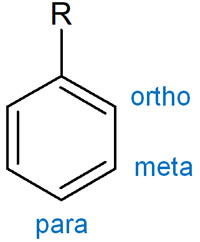
In the case of the benzene ring, the positions can be classified into three, starting from the substituent.
- The ortho position
- The meta position
- The para position
For example, electrophilic aromatic substitution reactions include Friedel-crafts reactions. When Friedel-crafts is performed on methoxybenzene (anisole), the following compounds are formed.
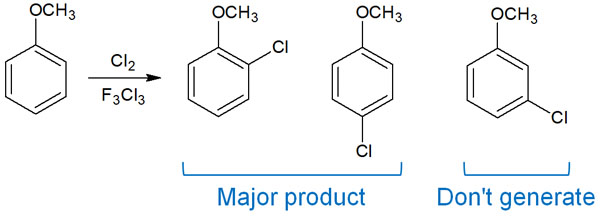
Electrophilic aromatic substitution reactions create a new substituent at the ortho or para position. Why is this selective for certain positions? Doesn’t a substitution reaction occur at the meta-position? This is because orientation is involved.
Resonance Effect by Substituents (R Effect)
When an aromatic compound undergoes an electrophilic substitution reaction, there are different types. Consider the following two types of benzene ring orientations.
- Ortho-para orientation
- Meta-orientation
So let’s understand that in the benzene ring, either the substitution reaction occurs at ortho-para, or the substitution reaction occurs at meta.
-Different People Have Different Ways of Explaining Orientation
Different people have different ways of explaining why they exhibit orientation. The following are some examples of the types of explanations.
- You can write a lot of resonance structures of reaction intermediates.
- You can write resonance structures that satisfy the octet rule in the reaction intermediates.
- There is repulsion (or stability) between charges in reaction intermediates.
- The resonance effect (R effect) is present.
These are all correct answers. In science, after experimental results are obtained, it is common to prepare a theory later in order to make sense of the results. In short, as long as you can understand it, any explanation is fine.
However, the easiest way to understand the orientation of aromatic compounds is to explain them using the resonance effect (R effect). The effect of molecular resonance is called the resonance effect (R effect). For example, aniline resonates as follows.
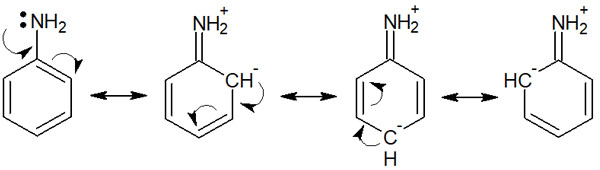
This resonance is caused by the influence of substituents present in the benzene ring. Once we understand the resonance, we can easily understand the orientation of the benzene ring.
The Orientation of Benzene Rings Varies with Electron-Donating and Electron-Withdrawing Groups
How does the resonance effect affect the orientation? In this regard, the orientation depends on whether the substituent on the aromatic compound is an electron-donating or an electron-withdrawing group.
For the resonance effect, the orientation of the benzene ring would be as follows.
- Electron-donating group: ortho-para orientation
- Electron-withdrawing group: meta-orientation
The substituents attached to the benzene ring can be divided into two groups: electron-donating and electron-withdrawing. Depending on whether the substituent is an electron-donating group or an electron-withdrawing group, the orientation of the benzene ring changes.
Why does this happen? The reason is that the electron density of the benzene ring varies from place to place as a result of resonance.
Phenol and Aniline Are Electron-Donating Groups: ortho-para Orientation
Typical functional groups that show electron-donating properties by binding to the benzene ring are as follows.
- Methoxy group (-OCH3)
- Hydroxy group (-OH)
- Amino group (-NH2)
The oxygen and nitrogen atoms in phenols and anilines act as electron-donating groups. When we draw the resonance structure, these aromatic ring compounds have higher electron density in ortho and para positions.
In the following, we again describe the resonance structure of aniline.

Thus, we can write resonances where electrons exist in the ortho and para positions. There are many electrons in the ortho and para, not in the meta.
In organic synthesis reactions, electrons attack other molecules to cause a reaction. By looking at these resonance structures, we can predict that when an electrophilic aromatic substitution reaction occurs, the electrons in the ortho or para positions will react chemically with other molecules. As a result, electrophilic substitution reactions occur in the ortho and para positions.
Earlier, we explained that in methoxybenzene (anisole), the substitution reaction occurs in ortho and para. This is because the electron-donating group bonds to the benzene ring, causing it to exhibit ortho-para orientation.
Orientation of ortho and para Involves Steric Hindrance
Of the ortho- and para-orientations, which is produced more, ortho- or para-? This is greatly affected by the substituents.
As you can see from the resonance structure, we can draw two resonances for ortho. On the other hand, para has only one resonance structure. Therefore, statistically speaking, it seems that the compound with a substituent in the ortho position would be produced double by the synthetic reaction.
In reality, however, this is not the case. In most cases, the substitution reaction occurs at the para position instead of the ortho position. Although a compound with a substituent in the ortho position is produced, there is a high probability that a compound with a substituent in the para position will be synthesized.
What is the reason for this? It is a steric hindrance. In the ortho position, the substitution reaction must be carried out with the substituent next to it. On the other hand, in the para position, steric hindrance does not occur because the distance from the substituent is far.
When you sit on a sofa, would you prefer to sit on a seat with a fat person in the middle? In that case, the sitting is very uncomfortable. If a fat person is sitting on the sofa, you will consciously avoid that sofa.

On the other hand, what if you find an empty sofa at a distance? You walk over to it and sit down, even if it’s far away.
The same is true for humans and substituents, both of which dislike three-dimensional obstacles. When a compound cannot physically enter a space because of a substituent, a steric hindrance occurs. As a result, the substitution reaction occurs in the para position instead of the ortho position. The larger the substituent, the more likely it is to cause steric hindrance.
Electron-Withdrawing Groups Such As Nitro and Carbonyl Groups Have meta-Orientation
On the other hand, what about electron-withdrawing groups? Electron-withdrawing substituents include, for example, the following.
- Carbonyl groups (-CO)
- Carboxy group (-COOH)
- Sulfone group (-SO3H)
- Nitro group (-NO2)
- Cyano group (-CN)
All of these functional groups have double or triple bonds. For these substituents with π-bonds, they will have electron-withdrawing properties.
In electron-withdrawing groups, they are meta-orientation. When an electrophilic substitution reaction occurs in an aromatic compound, we can assume that the substitution reaction occurs at the meta position, not at the ortho or para position. For example, nitrobenzene resonates as follows.
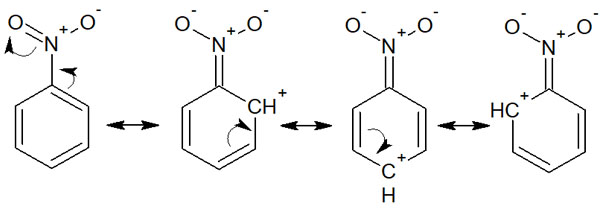
Considering the resonance effect, we can see that cations (positively charged carbons) are produced in the ortho and para positions. Due to the low probability of electrons, electrophilic substitution reactions are unlikely to occur at these positions. As a result, the substitution reaction occurs at the meta position where the electrons are present.
Sometimes Explained by the Stability of the Intermediate
Orientation by electron-withdrawing groups is often explained by the stability of the intermediate. For example, when a synthetic reaction occurs with nitrobenzene, the intermediates are as follows.
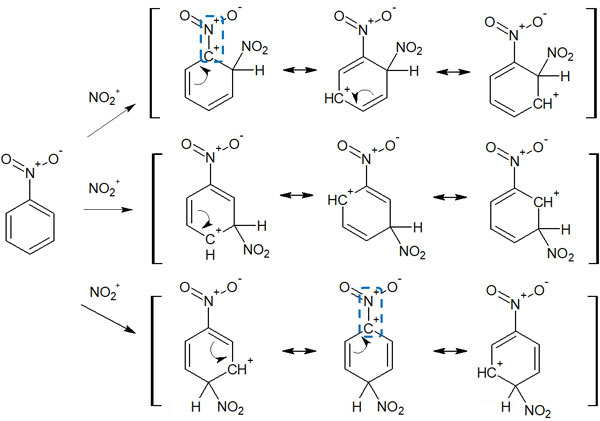
If a substitution reaction occurs at ortho or para, we can write the positive charges next to each other in the intermediate resonance, as noted in the figure above. When the same charges are next to each other, they repel each other. We can see that these resonance structures are unstable and do not contribute to stabilization by resonance.
On the other hand, what about when an electrophilic substitution reaction occurs in the meta position? The intermediate is not unstable, because there is no resonance structure with neighboring positive charges. The result is meta-orientation.
Some professors use this method to explain the meta-orientation of electron-withdrawing groups. It just makes it more difficult to understand. By writing all the resonance structures (ortho, meta, para), we can find the stable structure of the intermediate product. Therefore, the easiest way to understand the meta-orientation is by using the resonance effect (R-effect).
Halogens (Chlorine and Bromine) Have ortho- and para-Orientation
What happens in the case of halogens? There are a great many cases of aromatic compounds having halogens. The halogens that are important in organic chemistry are as follows.
- Fluorine (F)
- Chlorine (Cl)
- Bromine (Br)
- Iodine(I)
These halogens have ortho- and para-orientation. Halogens have unshared electron pairs (lone pair), which causes them to resonate. For example, in chlorobenzene, the resonance is as follows.
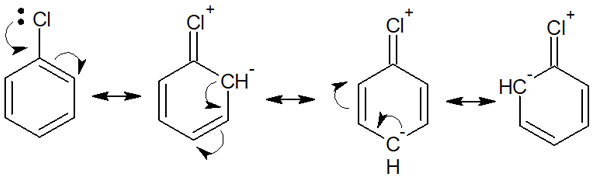
Chlorobenzene has ortho-para orientation, just as explained in the example of electron-donating groups. Halogen substituents, including fluorine, chlorine, bromine, and iodine, can be understood as ortho- and para-orientation.
Inductive Effect (I Effect) Reduces Reactivity
However, the properties of ordinary electron-donating groups and halogens are quite different. Halogens are known to have a remarkably high degree of electronegativity, so the electron density on the benzene ring is low. This is because the halogen attracts electrons to the ring. This is called the inductive effect (I effect).
The electron-donating group provides electrons through resonance, which increases the electron density on the benzene ring. Halogens, on the other hand, attract electrons.
It is somewhat confusing to a thing that it attracts electrons while giving them. This is because, in halogens, there are two completely different effects.
As mentioned above, the halogen substituents have ortho-para-orientation because of the resonance effect. The effect of resonance is usually stronger than electronegativity. Therefore, hydroxy (-OH) and methoxy (-OCH3) and amino (-NH2) groups are electron-donating groups and have a higher electron density in the benzene ring.
However, halogens are known for their strong electronegativity. The phenomenon that strongly attracts electrons due to electronegativity is the inductive effect. In halogens, the inductive effect is stronger, resulting in a lower electron density on the benzene ring and weaker reactivity.
- Resonance effect: higher electron density on the aromatic ring (ortho-para-orientation).
- Inductive effect: lower electron density on the aromatic ring.
In halogens, there are two completely different functions. As a result, although they exhibit ortho-para orientation, they are less reactive in electrophilic substitution reactions by attracting electrons on the benzene ring.
Alkylbenzene (Toluene) Has ortho-para Orientation
So far, we have examined the orientation of the various substitutions. However, some of the substituents are alkyl chains. If the alkyl chain is present in the aromatic ring, what happens to the orientation?
In alkylbenzene, the orientation is ortho-para. Alkyl chains are known to provide electrons. In other words, they are electron-donating groups. Although they do not have an unshared electron pair (lone pair) like oxygen and nitrogen atoms, and they do not actively resonate with each other, carbon atoms are known to be electron-donating groups that push out electrons.
It is important to understand that all electron-donating substituents exhibit ortho- and para-orientation.
Tertiary Carbocation Is Easily Stabilized
Why do alkylbenzenes exhibit ortho- and para-orientation? Carbon atoms do not resonate in the same way as oxygen and nitrogen atoms, although they provide electrons. Therefore, it is necessary to explain ortho-para orientation in a different way from the resonance effect.
This has to do with the stability of the carbocation. Although carbocation is an unstable substance, its stability varies with its structure. Among carbocations, the more carbon atoms are bonded to the carbocation, the more stable it is.
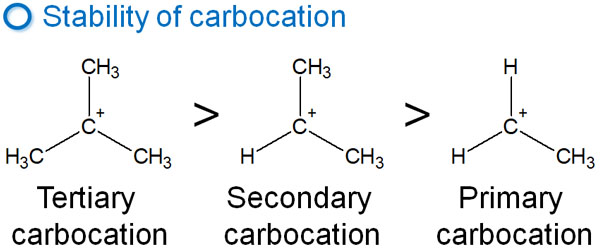
This difference in properties leads to differences in orientation in alkylbenzenes.
Let’s consider toluene as an example. A methyl group is attached to the benzene ring to form toluene. When an electrophilic aromatic substitution reaction occurs at toluene, the resonance of the intermediate is as follows.
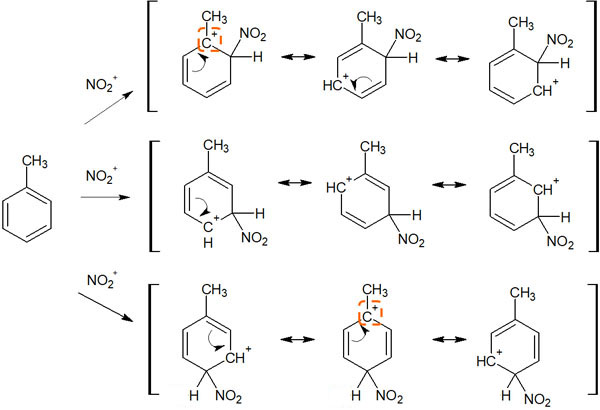
In the resonance structure of these intermediates, let’s focus on the carbocation state. Only when there is a substituent in the ortho and para positions, can we write the resonance structure of the tertiary carbocation. As a result, it is more stable than if the substitution reaction occurs at meta.
As mentioned earlier, tertiary carbocations are the more stable structures among carbocations. This is the reason why alkylbenzenes, such as toluene, show ortho- and para-orientation. If it is too hard to remember, understand that alkyl chains are electron-donating, and therefore ortho-para orientation is shown.
Reactivity Varies Greatly with Electron-Donating and Electron-Withdrawing Groups
We have explained that the orientation varies greatly depending on whether it is an electron-donating or an electron-withdrawing group. The electron-donating group results in ortho-para orientation, while the electron-withdrawing group results in meta-orientation.
Furthermore, the electronic state also has a significant effect on the reactivity of aromatic compounds. It is as follows.
- Electron-donating groups: higher reactivity.
- Electron-withdrawing groups: less reactive.
There are many electrons in the Benzene ring. This is why electrophilic substitution reactions occur. As you can see from the term “electrophilic substitution reaction,” it is difficult to carry out synthetic reactions if there are not many electrons on the benzene ring.
If an electron-donating group is present, electrons are actively pushed out into the benzene ring. Therefore, when an electron-donating group is present on the benzene ring, the synthetic reaction can be carried out with little energy.
On the other hand, the presence of an electron-withdrawing group makes the reaction less reactive. The electrons in the benzene ring are attracted to the functional group, so the benzene ring is no longer electron-rich. In the presence of electron-withdrawing groups, the activation energy required for a synthetic reaction is greater.

Depending on the electronic state of the benzene ring, the electrophilic substitution reaction will change.
Summary of Orientation and Reactivity (Reaction Rate)
How can we summarize the contents so far? The main points are as follows.
- Orientation changes with electron-donating groups and electron-withdrawing.
- Reactivity (rate of reaction) varies depending on whether there are more or fewer electrons in the benzene ring.
- Halogens have ortho and para orientation but attract electrons.
In an electrophilic aromatic substitution reaction, the following occurs.
| Substituent | Position | Reactivity |
| -NH2, -NHR, -NR2 | ortho and para | Very fast |
| -OH | ortho and para | Very fast |
| -OR (R=CH3 etc) | ortho and para | Very fast |
| -R (Alkyl chain) | ortho and para | Fast |
| -H | – | – |
| -F, -Cl, -Br, -I (halogen) | ortho and para | Slow |
| -COR (R=OH, OR, NH2 etc) | meta | Slow |
| -CN | meta | Slow |
| -SO3H | meta | Slow |
| -NO2 | meta | Slow |
Why does it have this orientation? And why does the reactivity (reaction rate) differ in this way? This can all be explained by electron-donating and electron-withdrawing groups.
Halogens are a special case; they have ortho- and para-orientation, but their reactivity is exceptionally poor. This is because of their high electronegativity, which causes an inductive effect.
Predicting Orientation from Substituents
By looking at the substituents, we can determine whether they are electron-donating or electron-withdrawing groups, and we can distinguish the orientation of the aromatic ring. For example, acetanilide has an electron-donating substituent.
At the nitrogen atom in the acetanilide, an unshared electron pair pushes electrons out to the benzene ring. The result is ortho-para-orientation by acting as an electron donor. They are also more reactive. Depending on the nature of the substituents, the orientation and reactivity of aromatic compounds can be predicted.
In the case of acetanilide, there is a substituent (-NH-CO-CH3). Since it is a large substituent, the steric hindrance is greater. Therefore, electrophilic substitution reactions are more likely to occur at the para position than at the ortho position.
Understanding Electrophilic Aromatic Substitution Reactions
Since the benzene ring contains a large number of electrons, it undergoes an electrophilic substitution reaction. This is called an electrophilic aromatic substitution reaction.
However, when a benzene ring undergoes an electrophilic substitution reaction, there is a law. The substitution reaction does not take place without control. The substitution reaction takes place in a specific position among ortho, meta, and para. The reason for this has been explained so far.
Some of the examples are special, like halogens. Also, in ortho-para-orientation, steric hindrance due to substituents has to be taken into account. By considering these factors, we can see what kind of compound synthesis is possible. We can also estimate the reactivity and reaction energy.
Reactions at the benzene ring are very important in organic chemistry. Understand these orientations and reactivities, and think about the synthetic reactions so as to obtain the desired compounds.