Every organic compound has a name. However, if you do not know the rules for naming them, you will not be able to find the compounds.
In such a case, there is a unified rule for naming compounds. This is called the IUPAC nomenclature. Let’s follow the IUPAC nomenclature to understand how to name compounds.
One of the first things you will learn in organic chemistry is the IUPAC nomenclature. However, the IUPAC nomenclature is less important. In organic chemistry, it is important to understand the reaction mechanisms, and there are no organic chemists who focus on the names of compounds.
Even so, if you don’t understand the rules of compound names, you will have trouble when looking for the reagents you want. So let’s learn the basic rules needed to name a compound.
Table of Contents
- 1 There Are Many Common Names, but Understand the Official Name
- 2 The Order in Which the Compounds Are Named
- 3 The Parent Chain Changes Depending on the Alkenes (Double Bonds) and Alkynes (Triple Bonds)
- 4 Nomenclature of Cyclic Compounds (Cycloalkanes)
- 5 IUPC Nomenclature for E/Z Isomers (cis-trans)
- 6 Understanding How Compounds Are Named
There Are Many Common Names, but Understand the Official Name
There are many common names for organic compounds; although they are not official names according to the IUPAC nomenclature, many people use them because they are widely used as common names.
Common names refer to a single substance. For example, the following compounds are famous for their common names.
- Acetaldehyde
- Ethyl acetate
- Phenol
- Glycerine
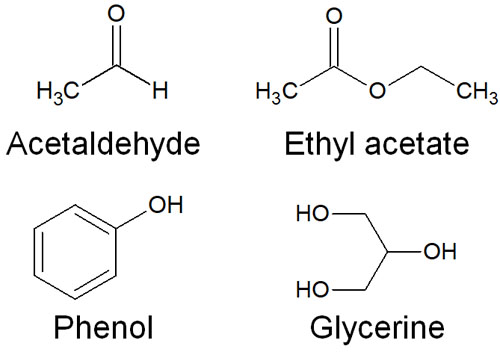
All of us who study chemistry have heard of these names. These are all common names and are not official names. However, many people use them, and no one uses the IUPAC nomenclature for these compounds.
Also, when searching for a compound (reagent), no one thinks of the IUPAC nomenclature and then searches for the compound. In fact, naming a compound using the IUPAC nomenclature from its structural formula is very complicated. So many people write down the structural formula of a compound and then use a tool that tells them the IUPAC nomenclature of the compound.
Alternatively, we often search for compounds using the number of atoms, such as carbon atoms, nitrogen atoms, oxygen atoms, and so on. After writing the structural formula, the tool or software will give you the molecular formula in an instant. You can then search for compounds with the target structural formula in the database.
More importantly, the IUPAC nomenclature is almost never used for compounds with complex structures. IUPAC nomenclature is generally used to find reagents for organic compounds, and many of these reagents have simple structures. You don’t need to be able to give the name of the compound in IUPAC nomenclature when you see a compound with a complex structural formula.
Considering this fact, IUPAC nomenclature is not a priority. However, if you do not have the knowledge, you will have trouble finding the reagents. In other words, it is not necessary to be able to use the IUPAC nomenclature perfectly, but it is necessary as knowledge.
The IUPAC Nomenclature of Organic Chemistry Has Rules
What are the rules of the IUPAC nomenclature? A common name applies to only one compound. Therefore, if a compound does not have a common name, we cannot know its name. On the other hand, if you use the IUPAC nomenclature, you can give a name to any compound. This is the difference between common names and IUPAC nomenclature.
In the IUPAC nomenclature, compounds are named according to rules. The rules are as follows.
- Prefix: Position and name of the substituent.
- Parent structure: The carbon skeleton of the parent chain.
- Suffix: The parent functional group.
Name the compounds in this order. For example, suppose you have the following compounds.
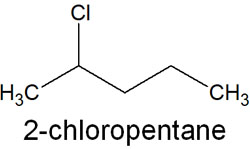
The IUPAC nomenclature for 2-chloropentane can be broken down as follows.
- Prefix: 2-chloro
- Parent structure: pent
- Suffix: ane
You should understand that it will always be divided into three parts. This is a simple rule when considering compound names in the IUPAC nomenclature.
The Order in Which the Compounds Are Named
How should we name compounds using the IUPAC nomenclature? There is an order to this.
The first thing to consider is the prefix and the parent structure; in IUPAC nomenclature, the prefix and the parent structure are the most difficult to consider. For these, you should consider the name of the compound in the following order.
- Determine the parent structure (parent chain)
- Number the parent chain.
- If there are multiple substituents, use Greek numerals.
- Arrange the substituents in alphabetical order.
We will explain the specific ideas below.
Look for the Longest Alkyl Chain First
When naming a compound according to the IUPAC nomenclature, the first thing you need to do is to find the parent structure of the compound. Organic compounds are made up of a carbon skeleton. Find the longest of these alkyl chains.
The longest chain of carbon is the parent structure. But in organic compounds, the carbon chains often branch out. So make sure you don’t mistake the main chain (parent chain).
For example, which is the longest carbon chain in the following compound?
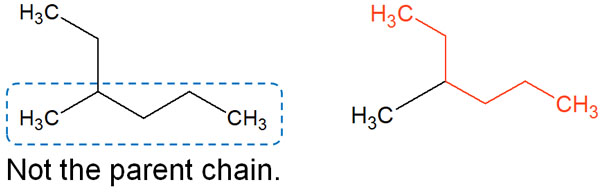
When you look at the structural formula, you would think that the five-carbon chain is the parent chain, as shown on the left side of the figure. However, as you can see on the right, the six-carbon chain is the longest. In other words, the parent structure of this molecule is an alkyl chain with six carbon atoms.
Look at the entire molecule and try to figure out where the longest carbon chain is. Here’s how the name of the parent structure changes depending on the number of carbon atoms.
| Number of carbon | Parent structure name | Name of alkane |
| 1 | meth- | methane |
| 2 | eth- | ethane |
| 3 | prop- | propane |
| 4 | but- | butane |
| 5 | pent- | pentane |
| 6 | hex- | hexane |
| 7 | hept- | heptane |
| 8 | oct- | octane |
| 9 | non- | nonane |
| 10 | dec- | decane |
For clarity, we have also added the names of the alkanes to the table. Alkanes have “ane” at the end. If you add “ane” to the name of the parent structure, it becomes the name of the alkane in the IUPAC nomenclature.
Look at the molecular skeleton and identify the longest carbon chain. For example, if you have a compound with seven carbons, you will find that you can use hept as the name of the carbon skeleton.
Assign Position Numbers to Substituents
The next step is to number the main chain. In many organic compounds, there are substituents. So, let’s number the substituent positions.
The rule is that the number should be as small as possible. For example, we can consider two different compound names in the following case.

In the IUPAC nomenclature, there is a rule to keep the numbers as small as possible. Therefore, the chloro group is in the second position, and 2-chloropentane is the correct name.
If There Are Multiple Substituents, Use Greek Numbers
What should we do if there are multiple substituents? If the same substituent is attached to the carbon skeleton, add a number to it. Use Greek numerals such as di and tri to indicate how many substituents are present.
The Greek numbers to use are as follows.
- Two: di
- Three: tri
- Four: tetra
- Five: penta
- Six: hexa
- Seven: hepta
The location number of the substituent should be smaller. In addition to that, if there is more than one of the same substituent, it is represented by a Greek number. For example, it would look like this.

For pentane, there are two chlorine groups attached in the second and third places, giving it the name 2,3-dichloropentane because of the two chlorine groups.
And what about the three fluorine groups in the second and third places for pentane? In this case, the compound shown in the above figure would be 2,3,3-trifluoropentane. In this way, Greek numerals are used to name the compounds.
Arrange the Substituents in Alphabetical Order
In contrast, it is common for different substituents to be attached to a compound. In that case, how should we think of the compound name? For this, let’s arrange them in alphabetical order.
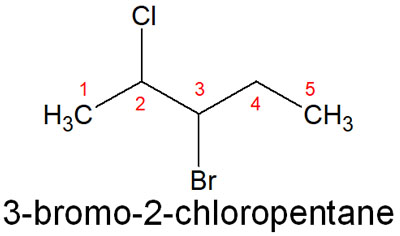
For this compound, 2-chloro-3-bromopentane is incorrect. Rather, it should be 3-bromo-2-chloropentane, which is in alphabetical order. In alphabetical order, b (bromo) comes first, rather than c (chloro).
You don’t have to think about the nature of the substituents; just arrange them in alphabetical order, so you don’t have to think hard.
Note that the numbers representing the number of substituents, such as di and tri, should not be included in alphabetical order. You need to name the compounds using the IUPAC nomenclature so that the names of the substituents are in alphabetical order.
The Parent Chain Changes Depending on the Alkenes (Double Bonds) and Alkynes (Triple Bonds)
So far, we have discussed the IUPAC nomenclature for alkanes. In alkanes, the longest carbon chain is the main chain.
However, not only do they have single bonds, but they can also be alkenes (double bonds) or alkynes (triple bonds). What happens with these alkenes and alkynes? For this, the parent chain changes.
The IUPAC nomenclature considers the main chain to contain as many double bonds (or triple bonds) as possible. Therefore, the longest carbon chain is not necessarily the parent chain. Although it is not the longest carbon chain, it can be the main chain because it contains many double bonds.
For example, think about the names of the following compound.
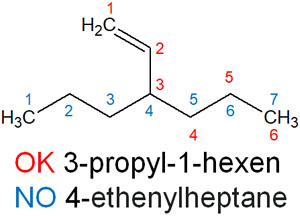
When considering the longest carbon chain, we must focus on the blue numbers. In this case, 4-ethenylheptane is the name of the compound.
However, this is not correct. You have to determine the main chain to contain more double bonds (or triple bonds). So, although the number of carbon chains is small, we need to focus on the red numbers to determine the parent chain. As a result, the compound name is 3-propyl-1-hexene.
In alkanes, ane is the last word. In alkenes, on the other hand, ene is the last word. In alkyn, it is yne. All you have to do is change the last word.
In alkenes and alkynes, it is necessary to note where the double or triple bond is located. So, indicate the location of the multiple bonds with a number, such as 3-propyl-1-hexene.
-Smaller Numbers Are Added to Double Bonds
Also, double bonds and triple bonds should be listed with as small numbers as possible. Focus on the double bonds (or triple bonds), not the substituents attached to the parent chain, and give them small numbers.
For example, suppose you have the following compound.
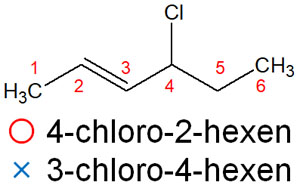
In this alkene compound, if you make the double bond number smaller, you will find the double bond in the second position. This is why it is called 4-chloro-2-hexene. On the other hand, if you try to make the number of the substituent smaller, you will get the compound name 3-chloro-4-hexene, which is wrong.
The Name of the Main Chain Depends on the Functional Group
Carbon chains have not only double and triple bonds, but also have various functional groups, such as hydroxy groups (-OH) and amino groups (-NH2). In addition, there is not only one functional group present, but several functional groups may be attached to a single carbon chain.
In this case, how should we think about it? Functional groups have a priority. There is a rule that if this functional group is present, the compound name should be considered with that functional group as the parent chain.
These functional groups have priority over alkenes and alkynes. In other words, the carbon chain with the functional group such as -OH or -NH2 attached to it has priority and becomes the main chain. The priority order of the functional groups is as follows.
| Priority | Type | Structure | Prefix | Suffix |
| 1 | carboxylic acid | -COOH | carboxy- | -oic acid |
| 2 | ester | -COOR | alkyl-oate | -oate |
| 3 | amide | -CONH2 | carbamoyl- | -amide |
| 4 | nitrile | -CN | cyano- | -nitrile |
| 5 | aldehyde | -CHO | formyl- | -al |
| 6 | ketone | -CO | oxo- | -one |
| 7 | alcohol | -OH | hydroxy- | -ol |
| 8 | amine | -NH2 | amino- | -amine |
| 9 | alkene | – | -ynyl | -ene |
| 10 | alkyne | – | -enyl | -yne |
| 11 | alkane | – | -yl | -ane |
There is no point in remembering these priorities; almost no organic chemist can accurately name them using the IUPAC nomenclature. And as mentioned above, the software can tell you the names of the compounds. Just as a matter of knowledge, you just need to understand that the priority is determined by the functional groups.
For example, suppose we have the following compound.
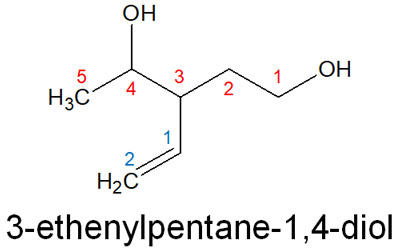
When comparing the double bond and the hydroxy group (-OH), the -OH has a higher priority. Therefore, the parent chain is the carbon chain to which the -OH is attached. As a result, the compound name is 3-ethenylpentane-1,4-diol.
We think of pentane as having an ethenyl attached to the 3rd carbon chain and -OH attached to the 1st and 4th carbon chains. This is why it is named as such.
Nomenclature of Cyclic Compounds (Cycloalkanes)
Some cyclic compounds exist. Cycloalkanes and cycloalkenes fall into this category. The nomenclature of cyclic compounds is simple if you understand what we’ve discussed so far. All you have to do is add cyclo- to the beginning. For example, the following are examples.
- Cyclohexane
- Cyclohexene
- Methylcyclopentane
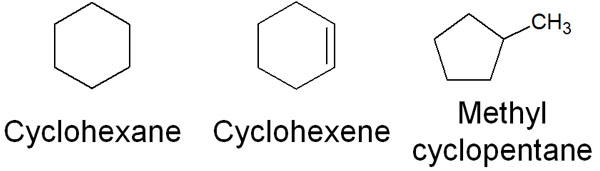
Note that if there is only one double bond or substituent, there is no need to number it. This is because the compound is symmetrical and is the same compound without numbering.
However, if there are two or more double bonds or substituents, you need to number the positions. The number of the double bond or substituent should be a small number. For example, it looks like this.

The order of preference and numbering of the substituents is the same as what we have explained so far. For cyclic compounds, just add cyclo- at the beginning.
Most of the cyclic compounds are related to cyclohexane (hexagon) or cyclopentane (pentagon). Other cyclic compounds have unstable structures, so these compounds should be considered as the main cyclic compounds.
IUPC Nomenclature for E/Z Isomers (cis-trans)
This is how we come up with the parent chain based on the number of hydrocarbons and functional groups, and then we note the names of the substituents in alphabetical order. However, the presence of a double bond often produces a cis-trans. These have to be identified because of the cis-trans result in completely different compounds.
However, the cis-trans you learned in high school chemistry is powerless. It is not possible to identify the compounds. For example, it is unclear which of the following compounds is cis/trans.
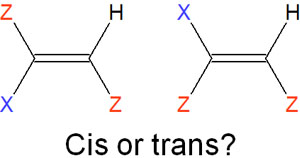
This is why the cis/trans nomenclature is ineffective. So we treat them as E/Z isomers. For the atoms attached to the double bond, we determine if they have a higher priority. This is as follows.
- Z (cis): Atoms of higher priority are on the same side.
- E (trans): Atoms of higher priority are on the opposite side.
The ranking of the substituents is simple: for E/Z isomers, the higher the atomic number of the atom bonded, the higher the priority. If the same atoms are bonded to each other, the order of precedence is determined by referring to the functional group (atomic number) bonded to each atom.
In the IUPAC nomenclature, we have explained the priority of the main chain, but in the E/Z isomers, the priority is determined by the size of the atomic number. Therefore, it is easy to determine. For example, suppose we have the following compound.

When comparing chlorine and bromine, the bromine atom has a higher atomic number.
Also, on the right side, the same carbon atoms are bonded together, but the ethyl group has a higher priority than the methyl group. Therefore, it is determined to be an E (trans); in IUPAC nomenclature, it is necessary to determine whether it is an E or Z.
Understanding How Compounds Are Named
There are many common names for organic compounds. However, it is not possible to use the common names for all compounds. This is because there is an infinite number of compounds. Therefore, the IUPAC nomenclature was created to give names according to rules.
In the IUPAC nomenclature, it is very difficult to predict the name of a compound with a complex structural formula. Also, each functional group has its own priority, and even double and triple bonds must be included. Therefore, many people draw the structural formula and then use the tool to figure out the name of the compound.
Therefore, there is no point in memorizing the IUAPC correctly and understanding it completely. However, it is essential that you understand the basics and be able to come up with the IUPAC names of simple compounds. Otherwise, you will not be able to search for reagents when performing organic synthesis.
Although you don’t need to memorize it hard at all, it is necessary to learn the knowledge of IUPAC nomenclature.
What we have described here is the basics of IUPAC nomenclature. You do not need to be able to guess the name of a complex compound. Instead, you should be able to find the name of a compound with a simple structural formula using IUPAC nomenclature.