It is common for some organic compounds to have double bonds. Instead of having only single bonds as an alkane, it is common for the molecule to be an alkene.
Double bonds can be created by organic synthesis. One way to do this is through elimination reactions. By reacting with a basic reagent, it is possible to create a compound with a double bond. This is called an elimination reaction.
There are three major types of elimination reactions, two of which are the E1 and E2 reactions. The E2 reaction is particularly important, and these synthetic reactions can be used to create alkenes.
What kind of synthetic reaction is an elimination reaction? We will explain the reaction mechanism and how it works.
Table of Contents
- 1 In the Elimination Reaction, a Double Bond Is Made from an Alkane: β Elimination
- 2 Reaction Mechanism of Alkenes by E1 Reaction
- 3 The E2 Reaction Is a One-Step Reaction, and the Nucleophile Is Involved in the Reaction Rate
- 4 E1 and E2 Reactions That Compete with SN1 and SN2 Reactions
- 5 E1cB Reaction in Which Anions Are Generated by Conjugation
- 6 Understand the Reaction Mechanism and Learn the Differences in Elimination Reactions
In the Elimination Reaction, a Double Bond Is Made from an Alkane: β Elimination
As mentioned earlier, the elimination reaction is a synthetic reaction that creates a double bond. It is a technique that is frequently used to make alkenes from alkanes.
Why is it called an elimination reaction, even though it is a synthetic reaction to make a double bond? The reason is that the double bond is formed by the elimination of a substituent in the molecule.
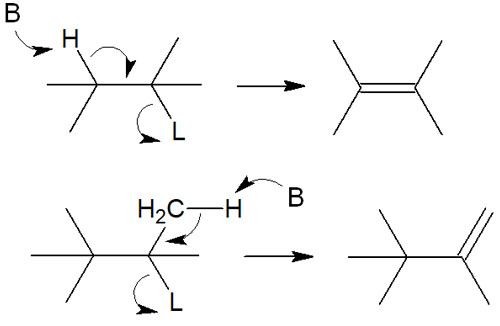
In the elimination reaction, the hydrogen is pulled out of the molecule by a basic substance. In addition, the leaving group attached to the adjacent carbon is also left. As a result, a double bond is formed. This kind of synthetic reaction in organic chemistry is an elimination reaction.
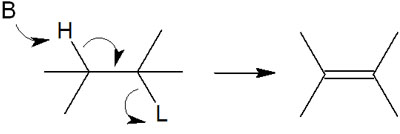
*B stands for Base and L for Leaving group.
If the carbon atom from which the hydrogen is removed is in the α-position, the neighboring carbon atom will be in the β-position. This reaction is called β-elimination because the leaving group in the β-position will be left.
There Are Three Types of Elimination Reactions
Note that there are three types of elimination reactions. It is common for a basic substance to pull out a hydrogen atom and forms a double bond while the leaving group is removed. However, the reaction mechanisms are different for each type of reaction. There are three types of reactions as follows
- E1 reaction: first, the leaving group is left, and then the hydrogen atoms are pulled out.
- E2 reaction: simultaneous leaving of a leaving group and withdrawal of a hydrogen atom.
- E1cB reaction: first, the hydrogen atom is removed and then the leaving group.
Each reaction differs in terms of when the hydrogen atoms are pulled out.
They are all the same in terms of creating a double bond. However, since the reaction mechanisms are different, they must be clearly separated and studied in organic chemistry.
Reaction Mechanism of Alkenes by E1 Reaction
Let’s start with the E1 reaction; for the E1 reaction to occur, the first step is the elimination of the leaving group. It is as follows.
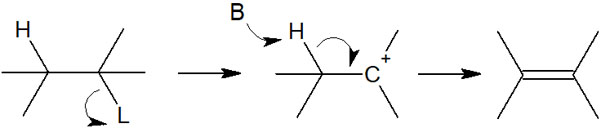
As the leaving group (L) leaves the molecule, it gives rise to a carbocation as an intermediate; in the E1 reaction, the reaction speed depends on how quickly the carbocation is produced, not on the basicity or concentration of the reagent.
As soon as the carbocation is formed, the hydrogen is withdrawn to make a double bond; in the E1 reaction, it is a two-step elimination reaction in which an intermediate is formed, and then a double bond is formed.
Stability of the Carbocation Is Important in the E1 Reaction
Since the carbocation is formed first in the E1 reaction, how stable the carbocation is has a great influence on the reaction rate.
There is no doubt that the carbocation is an unstable intermediate. However, if the intermediate is not stable to some extent, the intermediate will not be produced in the first place. It can be said that the E1 reaction proceeds because the intermediate carbocation is stable.
There is an order of stability for the carbocations. The order is as follows.
- Tertiary > Secondary > Primary
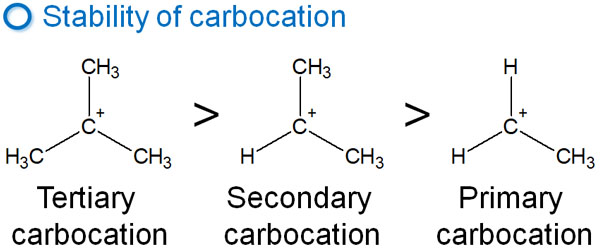
Primary carbocations and methyl carbocations are never produced. They are extremely unstable. Tertiary carbocations, on the other hand, are produced spontaneously. As for secondary carbocations, they are generated to a small extent.
Considering the E1 reaction, let’s understand that tertiary carbocations are the main ones. The E1 reaction proceeds at the carbocation, which has three alkyl chains attached to it.
Obtaining Compounds by the Saytzeff Rule
What kind of double bond is formed in the compound obtained in the E1 reaction? We explained earlier that alkenes are formed when hydrogen is extracted. However, due to the difference in the position from which the hydrogen is withdrawn, the following two types of compounds are produced.
Also, if you look at the molecules, one has two hydrogen atoms, and the other has six hydrogen atoms. Therefore, considering the probability, it can be assumed that the six hydrogen atoms have a higher probability of having hydrogen extracted from them.
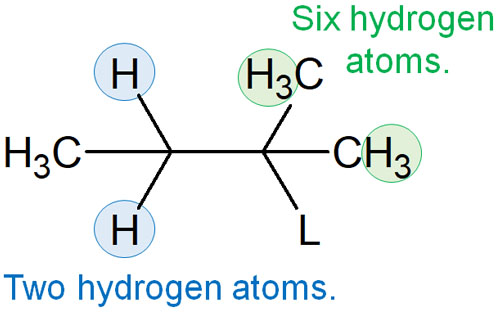
Moreover, with two hydrogen atoms, the base has to penetrate into the molecule in order to pull out the hydrogen atom. On the other hand, with six hydrogen atoms, the base can easily pull out the hydrogen atoms that exist outside the molecule. For this reason, it may seem that the synthetic reaction proceeds with six hydrogen atoms.
In fact, however, the E1 reaction proceeds with two hydrogen atoms. Why does this happen?
There is a natural law of organic chemistry called the Saytzeff rule. The Saytzeff rule refers to the law that when an alkene is made, the synthetic reaction proceeds in such a way that many substituents are present. As a result, alkenes with many substituents are synthesized.
The reason why the Saytzeff rule takes preference is that the transition state of the intermediate tends to be more stable. An alkene with more substituents requires less activation energy than an alkene with fewer substituents. Since less energy is required for the reaction, the synthesis of multisubstituted alkenes is preferred.
The Reason Why Multisubstituted Alkenes Are Stable Is Hyperconjugation
Why do alkenes with more substituents tend to be more stable? The reason for this is rarely explained in a straightforward manner.
Since the content is a bit more difficult, you can just remember, in the elimination reaction, multisubstituted alkenes are formed by the Saytzeff rule. So it is not a problem to skip this part. If you want to know the reason in more detail, you can study the mechanism of stability of multisubstituted alkenes.
In the stability of alkenes, the order is as follows.

In the Saytzeff rule, this order of stability is important. One of the reasons for this order is hyperconjugation. So what is hyperconjugation?
It is generally known that electrons are more easily stabilized when they are distributed over a wide range. All students of organic chemistry understand that if you can write conjugated structures, it will be stable. This is because electrons can exist in many places due to delocalization.
If there is a double bond, there will always be a π bond. A single bond is a σ bond; a bond in a perpendicular extension to a σ bond is a π bond.
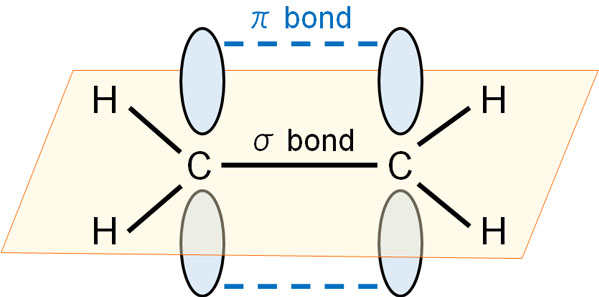
Even if there is a double bond, if it is a hydrogen atom that is attached to a carbon atom, it cannot interact with the carbon atom.
On the other hand, what if not a hydrogen atom but an alkyl chain is attached to the carbon atom as a substituent? In this case, the C-H bond and the pi bond of the double bond are parallel. As a result of the parallelization, they share each other’s electrons weakly.
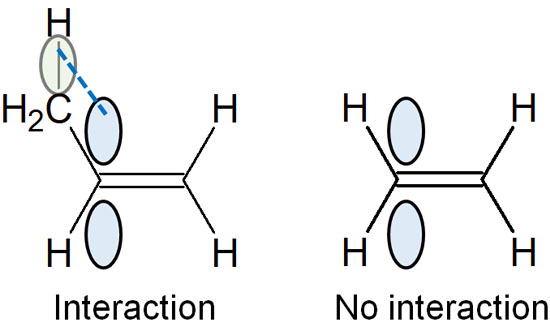
This is hyperconjugation. The electrons are not distributed over a wide area by writing resonance structures, as in a conjugate structure. It is just that the orbits are parallel, and the electrons are weakly shared, which is hyperconjugation. Due to the effect of hyperconjugation, it is more stable in alkenes with many substituents.
For this reason, the Saytzeff rule is followed when creating a double bond by elimination reactions.
In the Case of a Bulky Base, a Steric Hindrance Leads to Hofmann Elimination
By the way, some elimination reactions ignore the Saytzeff rule. Instead of making many substituents, the elimination reaction proceeds in such a way that it makes fewer substitutions. This is called Hofmann elimination.
When does a Hofmann elimination occur instead of the Saytzeff rule? It occurs when a bulky nucleophile (a base with a high steric hindrance) is used. Bulky bases include, for example, tert-butoxide.
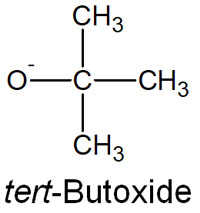
Unlike most bases, tert-butoxide is highly sterically hindered due to the large number of methyl groups attached to it. Using such a bulky nucleophile (base) makes it difficult for the reaction to follow the Saytzeff rule. As explained earlier, the hydrogen atoms inside the molecule have to be pulled out.
Because of the steric hindrance, hydrogen atoms inside the molecule cannot be pulled out. On the other hand, hydrogen atoms bond to the outside of the molecule can be pulled out without any problem, even with bulky nucleophiles.
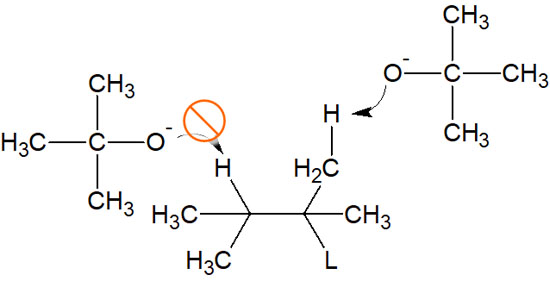
Usually, a reagent with less steric hindrance is used as the base. Therefore, the Saytzeff rule is followed. On the other hand, if a base with a high steric hindrance is used, an alkene with few substituents can be made by Hofmann elimination.
The E2 Reaction Is a One-Step Reaction, and the Nucleophile Is Involved in the Reaction Rate
On the other hand, how does the E2 reaction work? In the E2 reaction, the following things occur simultaneously.
- A nucleophilic agent (base) attacks a hydrogen atom.
- The leaving group is leaving.
In the E1 reaction, the first step is the elimination of the leaving group. In the E2 reaction, on the other hand, these reactions occur simultaneously to create a double bond. Therefore, the following reaction occurs at once.
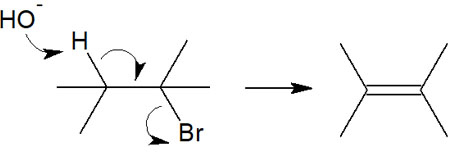
The stability of the carbocation is not relevant in the E2 reaction because no carbocation is formed. The E2 reaction occurs at all alkyl groups, tertiary, secondary and primary. Since there are no intermediates, the E2 reaction is a one-step elimination reaction.
Also, since the reaction occurs in a single step, the rate of the E2 reaction involves both the nucleophilic reagent (basic compound) and the reacting molecules.
Anti-Elimination (Antiperiplanar) Causes the Synthetic Reaction to Proceed
What is the key to the E2 reaction? It’s stereochemistry.
If a new double bond is created in a compound with an alkyl chain attached to it, then two types of compounds are created. These are so-called E/Z isomers, and there are two types: cis and trans. For this, which of the following compounds is produced in the E2 reaction?
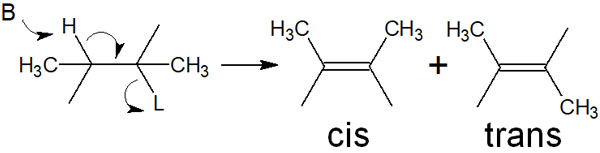
In order to understand this, you must learn two things
- The E2 reaction occurs in antiperiplanar.
- Anti-elimination occurs in an arrangement with less steric hindrance.
Let’s start by checking out the antiperiplanar. Considering the Newman projection, we can consider the following two conformations in stereochemistry.
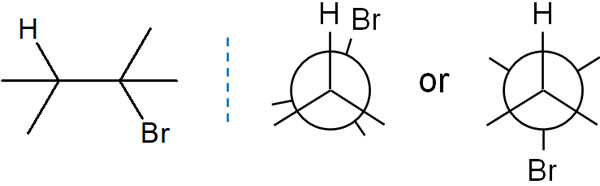
When a hydrogen atom and a bromine atom (a leaving group) are in the same position, it is called a synperiplanar. It is an overlapping form, and in this case, the energy state is high and unstable.
On the other hand, if the hydrogen atom and the bromine atom are on opposite sides, it is called an antiperiplanar. In this case, the atoms do not overlap each other and are not in a high energy (unstable) state. Moreover, only in the antiperiplanar state are the orbits perfectly parallel.
For this reason, E2 elimination proceeds in the antiperiplanar state. This is called anti-elimination.
Trans (E) Is Obtained to Reduce Steric Hindrance
We understand the reason for anti-elimination. So, which is produced, cis (Z) or trans (E)? In this regard, among the E/Z isomers, the trans (E) is mainly produced.
Considering the Newman projection, in the cis (Z), the methyl groups are located next to each other. As a result, the energy is higher due to steric repulsion.
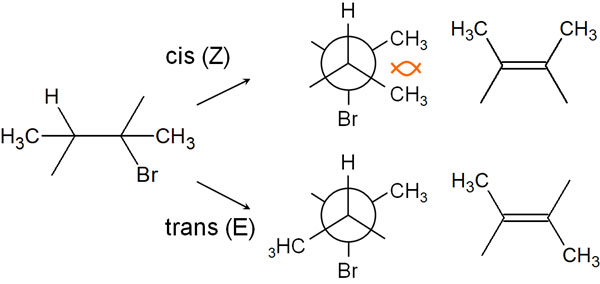
In contrast, what about trans (E)? In the case of trans, the methyl group is on the opposite side. Therefore, there is no steric repulsion, and it is easy for elimination reaction to occur in this state. Let’s understand that in the elimination reaction, the trans (E) product is mainly obtained.
Cyclohexane Undergoes an Elimination Reaction at the Axial Position
Understanding the stereochemistry in these elimination reactions will help us to understand the elimination mechanism of the E2 reaction in cyclohexane. Cyclohexane, also known as a cyclic compound, can take on two conformations. These are axial and equatorial.
The one in the horizontal position is equatorial. On the other hand, the one in the upper or lower position is axial. Of these, it is in the axial that the E2 reaction takes place.
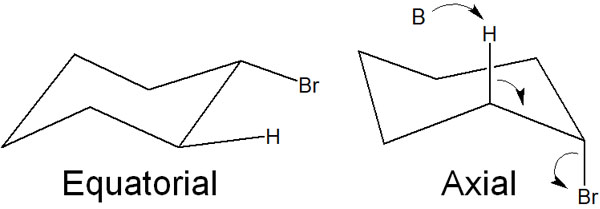
Equatorial does not allow for an antiperiplanar state. In other words, the E2 reaction does not occur. Therefore, the synthetic reaction always proceeds in an axial.
In the case of cyclic compounds such as cyclohexane, the Saytzeff rule may not be followed. For example, the following case is an example.
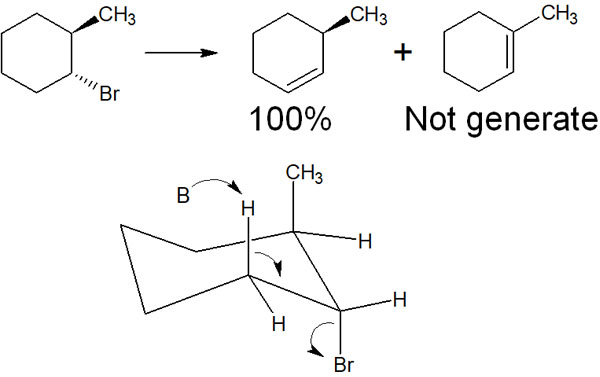
When performing a synthesis involving cyclohexene, there is only one hydrogen atom in the compound shown in the above figure that will be in the antiperiplanar position when it becomes axial. As a result, a compound is synthesized that does not follow the Saytzeff rule.
E1 and E2 Reactions That Compete with SN1 and SN2 Reactions
So far, we have reviewed the E1 and E2 reactions. In the E1 and E2 reactions, a double bond is created by the use of a nucleophile (base).
However, when nucleophiles are used, they can cause not only elimination reactions but also nucleophilic substitution reactions. The SN1 and SN2 reactions are known to be nucleophilic substitution reactions. In fact, both elimination reactions and nucleophilic substitution reactions often occur simultaneously.
In such cases, is it possible to distinguish which synthetic reaction will occur? In addition to the E1 and E2 reactions, we also need to check the SN1 and SN2 reactions. This can be summarized in the following table.
| Primary | Secondary | Tertiary | |
| Strong nucleophile | SN2 | SN2 | E1 or E2 or SN1 |
| Bulky nucleophile | E2 | E2 | E2 |
| Strong base | E2 (SN2) | E2 (SN2) | E2 |
| Weak base (Protic solvent) | – | SN1 or E1 | SN1 or E1 |
The important parts of the table above are noted in blue. When trying to predict which reaction will occur, focus on the blue text.
Depending on the reaction conditions, which reaction will proceed will vary. We will check why the synthetic reaction differs depending on the nucleophile used.
SN1 or SN2 Reactions Occur When There Is High Nucleophilicity and Little Steric Hindrance
When considering the reagents to be reacted with, the SN1 or SN2 reactions occur with reagents that are highly nucleophilic and have little steric hindrance. In the SN1 and SN2 reactions, the nucleophile must approach and attack the inside of the molecule as follows.
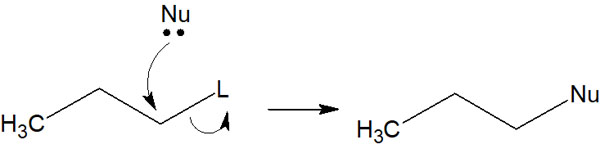
Therefore, smaller molecules (reagents with less steric hindrance) will cause SN1 or SN2 reactions.
For compounds with strong nucleophilicity, the nucleophilic substitution reaction will take precedence. Substances with strong nucleophilicity include the following.
- CN–
- RS–
- I–
In the case of these nucleophiles, the nucleophilic substitution reaction is the main process.
A Bulky Nucleophile or Strong Base Can Be Used in the E2 Reaction
On the other hand, when using a bulky nucleophile, the nucleophile is not able to get close to the inside of the molecule. As a result, an E2 reaction occurs.
We have already explained what happens in the case of bulky nucleophiles. In general, the E2 reaction follows the Saytzeff rule. However, due to the presence of steric hindrance, the reaction proceeds by Hofmann elimination when reagents such as tert-butoxide are used.
Even in the absence of such a bulky base, the E2 reaction is more likely to proceed when a strong base is used.
If the base is strong, the nucleophilic nature is also likely to be high. Therefore, the SN2 reaction also occurs at the same time. However, in general, strong bases attack hydrogen atoms. Rather than attacking the carbon atom to cause a nucleophilic substitution reaction, they prefer to pull the hydrogen atom out and make it stable.
Also, in a nucleophilic substitution reaction, a reagent has to go inside the molecule and attack the carbon atom. It is easier to attack the bare hydrogen atoms that exist outside the molecule. Therefore, the stronger the base, the more likely it is to cause an E2 reaction rather than a nucleophilic substitution reaction.
Especially for tertiary alkyl groups, the SN2 reaction does not occur due to steric hindrance. Therefore, only the E2 reaction occurs.
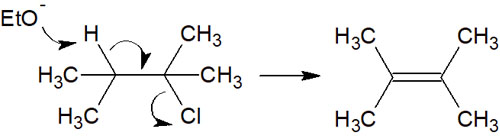
It is important to understand that the stronger the base used, and the higher the steric hindrance of the reaction compound, the more elimination reactions will occur.
However, if the molecular weight of the strong base reagent utilized is small and the steric hindrance of the reacting compound is low, the SN2 reaction may take preference over the E2 reaction. For example, sodium methoxide (CH3O–) and sodium ethoxide (CH3CH2O–) are strong bases, but very small molecules.
In this case, not only the strength of the base, but also the steric hindrance of the reacting compound, the temperature, the state of the compound, and all other factors come into play.
E1 and SN1 Reactions Occur at Weak Bases
On the other hand, a weak base may be used for synthesis. Weak bases include water, alcohol (methanol, ethanol), and acetic acid. These are also called protic solvents. Under these conditions, the E1 and SN1 reactions occur.
In the E2 and SN2 reactions, it is common to use reagents with strong nucleophilicity (strong basicity). However, in the E1 and SN1 reactions, the basicity of the nucleophile is not related to the rate of the reaction. Rather, the reactivity depends on how many carbocations are produced.
Therefore, the E1 and SN1 reactions proceed even when the base is weak. The hydrogen atoms of the carbocation are highly acidic, so even a weak base can pull out the hydrogen atoms.
-Both E1 and SN1 Reactions Occur
However, E1 and SN1 reactions cannot be separated. If we use protic solvent and react with a weak base, we can think that both the E1 and SN1 reactions will proceed to give two compounds. For example, the following.
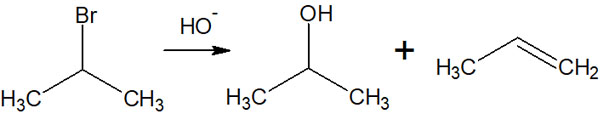
It is possible to change the likelihood of the E1 and SN1 reactions to occur depending on the reaction conditions; for example, when the temperature is increased, the E1 reaction is given priority. However, the fact that the two reactions proceed simultaneously does not change.
E1cB Reaction in Which Anions Are Generated by Conjugation
What we have discussed so far is the main part of the elimination reaction. In addition to this, we should also try to understand the E1cB reaction. The E1cB reaction is that the hydrogen atoms are first pulled out by the base to form an anion.
Under what conditions does the E1cB reaction occur? Let’s understand this reaction happens when a carbonyl group (-CO) is present near the leaving group. For example, the following.

In carbonyl groups, the acidity of the adjacent carbon (α-carbon) is known to be high. Therefore, the hydrogen atoms bonded to the α-carbon are pulled out by the base, resulting in the following reaction.

In short, consider that the hydrogen next to the carbonyl group is easily pulled out by the base and becomes an anion.
After becoming an anion in this way, the leaving group is released when the electrons return. As a result, a double bond is formed.
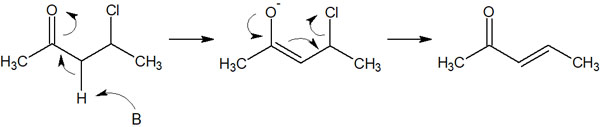
The cb in the E1cB reaction stands for a conjugate base. The base pulls out hydrogen (proton) and does not immediately form a double bond. Once, the anion is formed as a conjugate base. This reaction is called the E1cB reaction because it begins with deprotonation (the removal of a hydrogen atom).
The rate-limiting step in the E1cB reaction is the step where the leaving group is released. The proton next to the carbonyl group is highly acidic and can be easily extracted by a base. After that, when the electrons return, how quickly the leaving group is released is related to the reaction rate.
Incidentally, if there is a functional group that stabilizes the anion, such as a carbonyl group, the E1cB reaction always takes precedence. Under all conditions, it is important to understand that the E1cB reaction occurs before the E1, E2, SN1, and SN2 reactions.
Understand the Reaction Mechanism and Learn the Differences in Elimination Reactions
Two very important synthetic reactions in organic chemistry are the SN1 and SN2 reactions. However, once you have learned about these nucleophilic substitution reactions, in many cases, you will be learning about elimination reactions.
There are many similarities between nucleophilic substitution reactions and elimination reactions. And as we have discussed, these reactions often occur simultaneously. So we have to determine under what conditions the reaction takes precedence.
Also, even in the same elimination reaction, different nucleophiles (bases) will change the compounds produced. Normally, the reaction is by the Saytzeff rule, but it can also be a Hofmann elimination.
Be sure to understand the differences in these reaction mechanisms. Once you have learned the three types of elimination reactions and understand how the ease of the reaction changes depending on the reagent (nucleophile) you use, you will be able to synthesize alkenes whenever you want.