Organic compounds often have benzene rings, and synthetic reactions of aromatic compounds are very important. Aromatic rings are highly stable and usually do not react chemically.
In some cases, however, it is possible to replace substituents on the aromatic rings. This is called nucleophilic aromatic substitution. If a leaving group is present in the benzene ring, it can be replaced by another substituent by reacting with a nucleophilic reagent.
The most well-known nucleophilic aromatic substitution is the Sandmeyer reaction. Any substituent can be synthesized via a diazonium salt. Other nucleophilic aromatic substitution reactions include synthetic reactions using halogens and benzynes.
What are the reaction mechanisms of nucleophilic aromatic substitution reactions? And what kind of substituents can be added? We will discuss these questions.
Table of Contents
Nucleophilic Aromatic Substitution Reactions in which the Substituent of the Benzene Ring Is Replaced
In most compounds with aromatic rings, substituents are attached to them. Some of these substituents may undergo a substitution reaction in the presence of a nucleophilic reagent.
In a nucleophilic substitution reaction, a leaving group must be present. When the nucleophilic reagent (Nu) attacks the molecule, the leaving group (L) is replaced. Nucleophilic substitution reactions occur on the benzene ring by the following reaction mechanism.
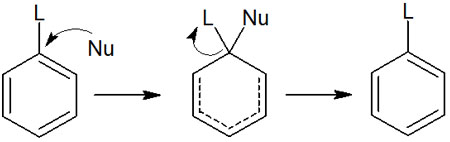
As in general nucleophilic substitution reactions for alkanes, the substituent is replaced on the same carbon atom. Therefore, the reaction mechanism is simple.
Substitution Reactions to Halogens Occur on Aromatic Rings
One of the simplest nucleophilic aromatic substitutions is the substitution of a halogen. In nucleophilic substitution reactions for alkyl chains, the halogen becomes a leaving group. Similarly, the halogen on the benzene ring also becomes a leaving group and is attacked by nucleophilic reagents.
For example, by reacting with an aromatic ring containing a halogen under basic conditions, the halogen is replaced by -OH. As a result, phenol can be synthesized.
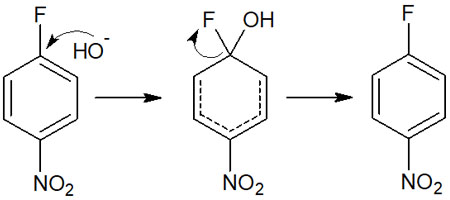
In this way, the halogen can be changed to another substituent.
Of course, if you change the reaction conditions, you can get different compounds. For example, if you react with ammonia instead of reacting with water, you get aniline instead of phenol. It is possible to synthesize amines.
Note that the leaving groups of halogens are easy to be released in the following order.
- F (fluorine) > Cl (chlorine) > Br (bromine) > I (iodine)
The reactivity of halogens is opposite to that of SN1 and SN2 reactions for alkyl chains. In the nucleophilic substitution reaction of alkyl chains, the more easily the leaving group becomes an ion, the easier it is to react. Therefore, iodine, which has a weak carbon-halogen bond, has the highest reactivity.
On the other hand, for halogens on the benzene ring, attack and addition by nucleophiles is the rate-limiting step. Since atoms with less steric hindrance are easier to leave, the fluorine atom is the best leaving group.
Halogens Are Replaced by the Presence of Electron Withdrawing Groups or Pyridines
Note that aromatic rings are stable, and the synthesis does not proceed under normal conditions. However, in the presence of electron-withdrawing groups, halogen substitution reactions can exceptionally take place. The following are known to be electron-withdrawing groups.
- Nitro group (-NO2)
- Cyano group (-CN)
- Carbonyl group (-CO)
- Sulfo group (-SO3H)
Why does the presence of these substituents activate the benzene ring and lead to nucleophilic substitution reactions? This is because the presence of electron-withdrawing groups allows us to write the following resonance structure.
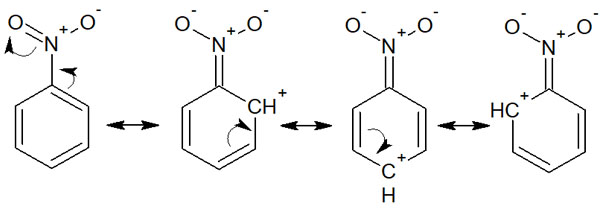
We can see that the ortho and para positions from the electron-withdrawing group have positive charges. This makes it easier for nucleophiles with a negative charge to attack them, and halogen substitution is more likely to occur.
As we have explained, the presence of an electron-withdrawing group on the benzene ring makes it exceptionally possible to carry out nucleophilic aromatic substitutions of halogens. For reference, this reaction mechanism is called SNAr.
-The Reaction Also Occurs with Heterocyclic Compounds such as Pyridine
It is not only the effect of the electron-withdrawing group that causes the aromatic ring to be positively charged due to the resonance structure, but the same is true for heterocyclic compounds.
A pyridine with a nitrogen atom in the benzene ring has a lower electron density on the benzene ring, just like electron-withdrawing groups. This is because we can write the following resonance structure.
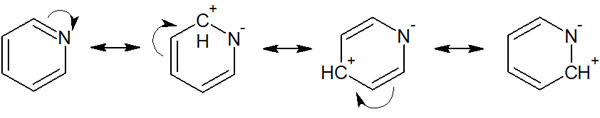
Therefore, nucleophilic aromatic substitution reactions occur not only with electron-withdrawing groups but also with heterocyclic compounds such as pyridines.
Sandmeyer Reaction with Diazonium Salts Is the Most Common
What is the most common nucleophilic aromatic substitution? The most commonly used nucleophilic aromatic substitution is the Sandmeyer reaction.
In the halogen substitution reaction just described, the reaction cannot proceed unless an electron-withdrawing group is present on the benzene ring. On the other hand, in the case of the Sandmeyer reaction in which diazonium salts are formed, nucleophilic aromatic substitution can occur regardless of the substituents present on the aromatic ring.
When sodium nitrite (NaNO2) is added to aniline under acidic conditions, the diazonium salt is formed. Under acidic conditions, sodium nitrite becomes a nitrosonium ion, as shown below.
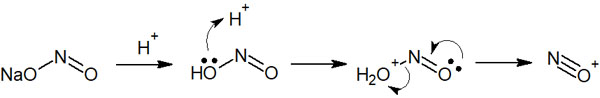
Subsequently, aniline reacts with a nitrosonium ion to form a diazonium compound. The reaction mechanism is as follows.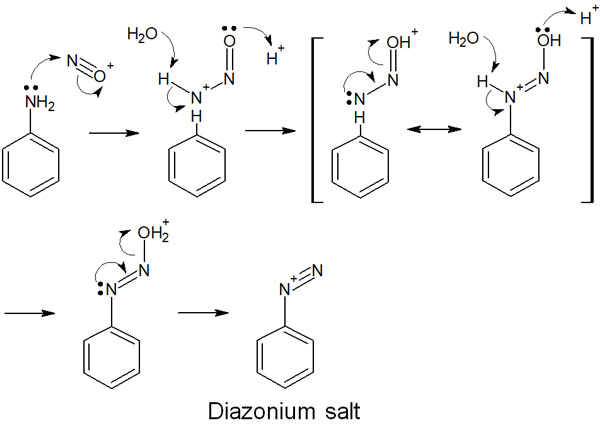
Diazonium salts are known to be strong leaving groups. Therefore, when a nucleophile is added to a diazonium compound, a nucleophilic aromatic substitution reaction occurs.
For example, phenol can be synthesized by reacting a diazonium compound with water.
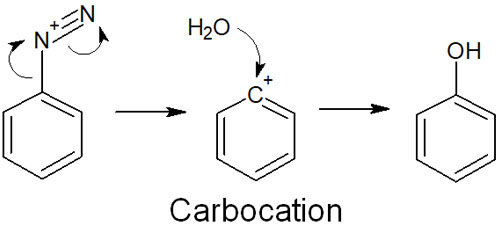
In diazonium salts, the phenyl cation is formed as an intermediate. The phenyl cation is extremely unstable and is attacked by nucleophiles. As a result, substitution reactions occur.
Any Substituents Can Be Put in via Diazonium Compounds
Why is the Sandmeyer reaction the most important nucleophilic aromatic substitution? It is because many substituents can be put in via diazonium compounds.
In the previous example, we described the synthesis of phenol by reacting with water. By changing the reagent to be reacted, different substituents can be added as follows.
| Reagents | Substituents generated |
| CuCl | Cl (chlorine) |
| CuBr | Br (bromide) |
| KI | I (iodine) |
| H2O | OH (phenol) |
| CuCN | CN (cyano group) |
| BF4– | F (fluorine) |
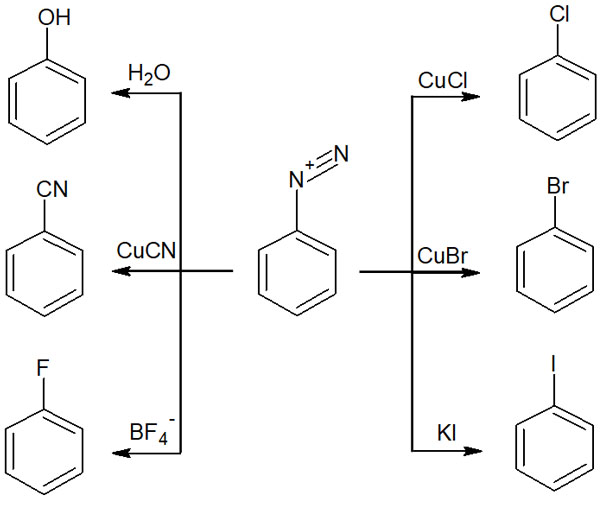
Of course, there are many other types of nucleophiles. For example, alcohols and thiols act as nucleophiles and cause nucleophilic aromatic substitution reactions. In any case, any molecule that has nucleophilic properties, even slightly, including water, causes a nucleophilic aromatic substitution with diazonium compounds.
The Sandmeyer reaction using diazonium compounds is the most important nucleophilic aromatic substitution because you can synthesize any substituent.
Nucleophilic Aromatic Substitution Reaction via Benzyne
When studying nucleophilic aromatic substitution reactions, the reaction mechanism of benzyne is often used as an example. The reaction mechanism is different from the Sandmeyer reaction, and a substituent can be changed.
A compound that has a triple bond on the benzene ring is called benzyne. Nucleophilic aromatic substitution reactions using benzynes are possible when fluorine or chlorine atoms are present on the benzene ring, such as in fluorobenzene and chlorobenzene.
When a strong base is added to fluorobenzene or chlorobenzene, the hydrogen (proton) is withdrawn, and benzyne is formed. If a nucleophilic agent such as ammonia is present in the solution, the nucleophilic agent attacks the benzyne to form a substituent.

Strictly speaking, the synthetic reaction of benzyne is different from the nucleophilic aromatic substitution. The first reaction is the elimination, which produces benzyne. Subsequently, aniline is synthesized by the addition of ammonia.
But overall, a substitution reaction is taking place. This is why we learn synthetic reactions with benzyne in nucleophilic aromatic substitution.
Learn the Properties of Substitution Reactions on Aromatic Rings
The benzene ring is a highly stable substance and therefore does not normally cause a reaction. However, under certain conditions, substitution reactions can occur with aromatic rings.
There are only three important nucleophilic aromatic substitution reactions. They are as follows.
- Halogen substitution reactions
- Sandmeyer reaction
- Reaction via benzyne
The most important of these is the Sandmeyer reaction. It is one of the most frequent synthetic reactions, so most laboratories that perform organic synthesis use diazonium salts. Therefore, it is particularly important to understand the reaction mechanism of the Sandmeyer reaction.
The nucleophilic aromatic substitution is useful when you want to add a new substituent to the benzene ring. Be sure to understand the mechanism of the reactions so that you can proceed with the synthetic reactions.